Alerginol Plus film-coated tablets blister No. 10
Instructions for use Allerginol Plus film-coated tablets, blister pack No. 10
Composition
active ingredient: montelukast, levocetirizine dihydrochloride;
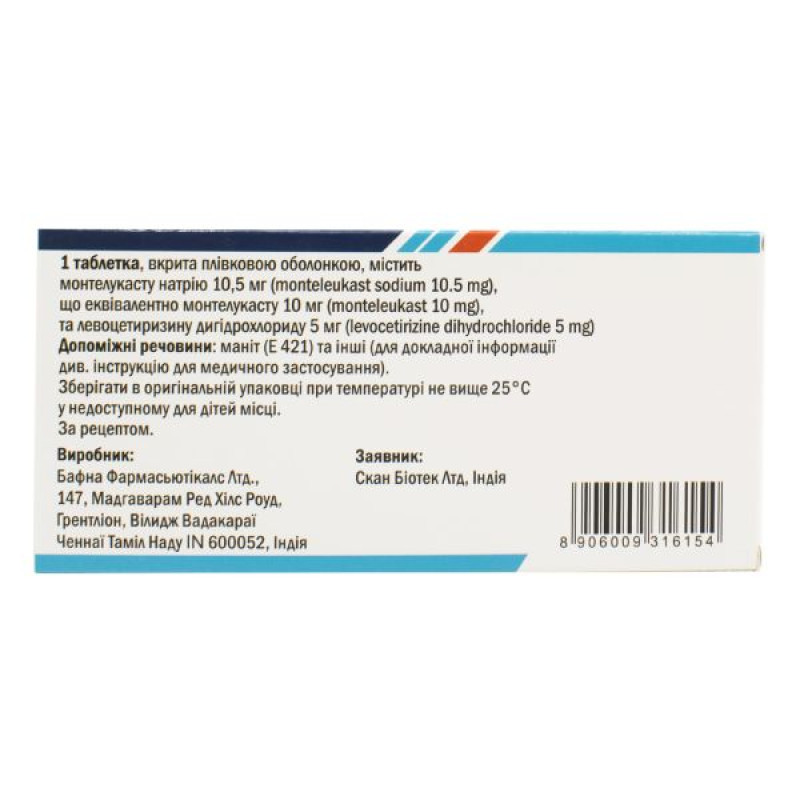
1 film-coated tablet contains montelukast sodium 10.5 mg, equivalent to montelukast 10 mg, and levocetirizine dihydrochloride 5 mg;
Excipients: microcrystalline cellulose, mannitol (E 421), croscarmellose sodium, hydroxypropylcellulose, magnesium stearate, Opadry white (polyethylene glycol, titanium dioxide (E 171), hypromellose).
Dosage form
Film-coated tablets.
Main physicochemical properties: white, biconvex, round tablets, film-coated, smooth on both sides.
Pharmacotherapeutic group
Antiasthmatics. Selective and orally active leukotriene receptor blocker. ATX code R03DC03.
Antihistamines for systemic use. Piperazine derivatives. ATC code R06AE09.
Pharmacological properties
Pharmacodynamics.
Montelukast.
Cysteinyl leukotrienes (LTC4, LTD4, LTE4) are potent inflammatory eicosanoids secreted by various cells, including mast cells and eosinophils. These important pro-asthmatic mediators bind to cysteinyl leukotriene receptors (CysLT) present in the human airways (including smooth muscle myocytes and macrophages) and other pro-inflammatory cells (including eosinophils and some myeloid stem cells). CysLTs are implicated in the pathophysiology of asthma and allergic rhinitis. In asthma, leukotriene-mediated effects cause bronchospasm, sputum production, increased vascular permeability, and increased eosinophil numbers. In allergic rhinitis, CysLT is released from the nasal mucosa during hypersensitivity reactions following allergen exposure and is manifested by the symptoms of allergic rhinitis. An intranasal challenge with CysLT has demonstrated an increase in nasal airway resistance and symptoms of nasal obstruction.
Montelukast sodium is an active compound that binds with high selectivity and chemical affinity to CysLT1 receptors. Montelukast inhibits the physiological action of LTD4 at CysLT1 receptors without showing affinity for the receptors.
Levocetirizine hydrochloride.
Levocetirizine, the R-enantiomer of cetirizine, is a potent and selective antagonist of peripheral H1-histamine receptors. Binding studies have shown that levocetirizine has a high affinity for human H1-histamine receptors (Ki=3.2 nmol/l). The affinity for H1-histamine receptors of levocetirizine is 2 times higher than that of cetirizine (Ki=6.3 nmol/l). Levocetirizine dissociates from H1-histamine receptors with a half-life of 115 minutes ± 38 minutes. After a single dose of levocetirizine, receptor occupancy is 90% after 4 hours and 57% after 24 hours. In placebo-controlled trials using a chamber model of allergic reaction, levocetirizine at a dose of 5 mg began to suppress pollen allergy symptoms 1 hour after administration. In vitro studies (Boyden chamber and cell layer methods) have shown that levocetirizine inhibits the activity of eotaxin-induced transendothelial migration of eosinophils in skin and lung cells. In vivo pharmacodynamic studies (skin chamber method) have demonstrated three main inhibitory effects of levocetirizine at a dose of 5 mg in the first 6 hours after pollen exposure compared to placebo in 14 adult patients: inhibition of VCAM-1 (vascular endothelial adhesion molecule type I) release, changes in vascular permeability and reduction of eosinophil activity. Studies have shown that the activity of levocetirizine at half the dose is comparable to that of cetirizine on both skin and nasal mucosa. The pharmacokinetic/pharmacodynamic relationship of levocetirizine at a dose of 5 mg is similar to the pattern of inhibition of the histamine-induced "bloom" reaction by cetirizine at a dose of 10 mg. As with cetirizine, the effect on histamine-induced skin reactions is independent of plasma concentrations. ECG data did not show a relevant effect of levocetirizine on the QT interval.
Pharmacokinetics.
Montelukast.
Distribution: Montelukast is more than 99% bound to plasma proteins. The volume of distribution of montelukast at steady state averages 8 to 11 liters. In studies in rats, the passage of radiolabeled montelukast across the blood-brain barrier was minimal. In all other animals, radiolabeled material concentrations were also minimal 24 hours after dosing.
Metabolism: Montelukast is extensively metabolized. Steady-state plasma concentrations of montelukast metabolites have not been determined in studies with therapeutic doses in adults and pediatric patients.
In vitro studies using human liver microsomes have shown that cytochromes P450 3A4 and 2C9 are involved in the metabolism of montelukast. Clinical studies of the effects of known inhibitors of cytochromes P450 3A4 (e.g., ketoconazole, erythromycin) or 2C9 (e.g., fluconazole) on the pharmacokinetics of montelukast have not been conducted. Further in vitro studies using human liver microsomes indicate that montelukast does not inhibit cytochromes P450 3A4, 2C9, 1A2, 2A6, 2C19, and 2D6 at therapeutic concentrations. In vitro studies have shown that montelukast is a potent inhibitor of cytochrome P450 2C8; however, data from a clinical drug interaction study with montelukast and rosiglitazone (a marker drug substrate primarily metabolized by CYP2C8) indicated that montelukast does not inhibit CYP2C8 in vitro and therefore should not alter the metabolism of drugs metabolized by this enzyme.
Elimination: The plasma clearance of montelukast in healthy adults averages 45 mL/min. Following an oral dose of radiolabeled montelukast, 86% was recovered in the feces within 5 days and less than 0.2% in the urine. This, together with the oral bioavailability of montelukast, indicates that its metabolites are almost entirely eliminated in the bile. The mean plasma elimination half-life of montelukast in young healthy subjects is 2.7 to 5.5 hours. The pharmacokinetics of montelukast are linear over oral doses up to 50 mg. A small amount of accumulation (14%) of the active substance in plasma was observed after administration of 10 mg of montelukast once daily.
Levocetirizine hydrochloride.
The pharmacokinetics of levocetirizine are linear, independent of dose and time, and have little interpatient variability.
Absorption. Levocetirizine is rapidly and extensively absorbed after oral administration. The maximum plasma concentration is reached 0.9 hours after administration. Steady-state concentrations are reached after 2 days. Cmax is 270 ng/ml after a single dose and 308 ng/ml after repeated administration at a dose of 5 mg, respectively.
The extent of absorption of the drug is independent of dose and does not change with food intake, but the maximum concentration of the drug decreases and reaches its maximum value later.
Distribution: There is no information on the distribution of levocetirizine in human tissues, nor on the penetration of levocetirizine through the blood-brain barrier. Levocetirizine is 90% bound to plasma proteins. The distribution of levocetirizine is limited, as the volume of distribution is 0.4 l/kg.
Biotransformation. In humans, about 14% of levocetirizine is metabolized, so differences due to genetic polymorphism or concomitant use of enzyme inhibitors should be insignificant. The metabolic process includes aromatic oxidation, N- and O-dealkylation and conjugation with taurine. Dealkylation occurs primarily with the participation of CYP 3A4, while the aromatic oxidation process involves multiple and/or unidentified CYP isoforms. Levocetirizine does not affect the activity of cytochrome isoenzymes CYP 1A2, 2C9, 2C19, 2D6, 2E1, 3A4 at concentrations significantly exceeding the maximum after taking a 5 mg oral dose. Given the low degree of metabolism and the lack of ability to inhibit metabolism, the interaction of levocetirizine with other substances is unlikely.
Elimination. The plasma half-life of the drug in adults is 7.9 ± 1.9 hours. The average total clearance is 0.63 ml/min/kg. Levocetirizine and its metabolites are mainly excreted in the urine (an average of 85.4% of the administered dose is excreted). Only 12.9% of the administered dose is excreted in the feces. Levocetirizine is excreted mainly by glomerular filtration and active tubular secretion.
Indication
For the relief of symptoms associated with seasonal and perennial allergic rhinitis, as well as rhinitis in patients with bronchial asthma.
Contraindication
Hypersensitivity to montelukast sodium, levocetirizine or cetirizine, as well as to other components of the drug. The drug is also contraindicated in severe renal failure (creatinine clearance < 10 ml/min); in severe hereditary galactose intolerance, lactase deficiency or glucose-galactose malabsorption. Children under 15 years of age.
Interaction with other drugs and other types of interactions
In drug interaction studies, the recommended clinical dose of montelukast had no clinically significant effect on the pharmacokinetics of the following drugs: theophylline, prednisolone, oral contraceptives (norethidrone 1 mg/ethylestradiol 35 mg), terfenadine, digoxin, and warfarin. Although specific drug interaction studies with montelukast have not been conducted, in several studies, montelukast has been coadministered with many commonly prescribed drugs without any clinically significant interactions. These drugs include thyroid hormones, sedatives and hypnotics, nonsteroidal anti-inflammatory drugs, benzodiazepines, and decongestants.
In patients receiving concomitant phenobarbital, which induces hepatic metabolism, the area under the concentration-time curve (AUC) for montelukast following a single 10 mg dose was decreased by approximately 40%. However, no dose adjustment of montelukast is recommended. Concomitant use of montelukast with potent cytochrome P450 inhibitors such as phenobarbital or rifampin should be done under appropriate clinical supervision.
Levocetirizine hydrochloride.
In vitro data indicate that levocetirizine does not cause pharmacokinetic interactions by inhibition or induction of hepatic enzymes that metabolize the drug. In vivo drug interaction studies with levocetirizine have not been performed. Pharmacokinetic drug interaction studies conducted with racemic cetirizine have shown that cetirizine does not interact with antipyrine, pseudoephedrine, erythromycin, ketoconazole and cimetidine. A small decrease in cetirizine clearance (16%) was observed with 400 mg theophylline. It is likely that higher doses of theophylline may potentiate this effect. Ritonavir increased cetirizine plasma concentrations by approximately 42%, increased the elimination half-life by 53% and decreased cetirizine clearance by 29%. Ritonavir disposition was not altered by co-administration of cetirizine.
Application features
Montelukast.
Montelukast is not indicated for the relief of bronchospasm in acute asthma attacks, including status asthmaticus. Patients should be advised to use appropriate treatment in the event of such attacks. Montelukast therapy may be continued during acute asthma attacks. Montelukast should not be abruptly substituted for inhaled or oral corticosteroids; the dose of inhaled corticosteroids may be gradually reduced under medical supervision.
Montelukast should not be used as monotherapy for the relief of exercise-induced bronchospasm. If acute asthma attacks occur after exercise, patients should continue to use their regular inhaled β-agonists as a prophylactic agent and carry a short-acting inhaled β-agonist for emergency use.
Montelukast treatment does not allow patients with aspirin-dependent asthma to use aspirin or other nonsteroidal anti-inflammatory drugs.
Eosinophilia: In isolated cases, patients receiving montelukast may experience systemic eosinophilia, sometimes with clinical manifestations of vasculitis, the so-called Churg-Strauss syndrome, which is treated with systemic corticosteroid therapy. Such cases have usually been associated with a reduction in the dose of oral corticosteroids.
No differences in safety and efficacy have been observed between elderly and younger patients, although increased sensitivity to the drug in some elderly patients cannot be ruled out.
Since montelukast is primarily excreted in the bile, this combination product should be administered with caution to patients with hepatic impairment.
Levocetirizine hydrochloride
During the use of the drug, you should refrain from drinking alcohol. The drug is not recommended for patients with severe hereditary galactose intolerance, lactase deficiency or glucose-galactose malabsorption.
Clinical studies of levocetirizine in each approved indication have not been conducted in sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. Other clinical studies have also not demonstrated any differences in response between younger and older patients. In general, elderly patients should be treated with caution and should be started at the lowest dose, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or use of other medications.
Since levocetirizine is excreted primarily in the urine, patients with renal insufficiency may require dosage adjustment. This combination product should be used with caution in such patients.
Use during pregnancy or breastfeeding
Since there are no adequate and well-controlled studies on the safety of montelukast or levocetirizine during pregnancy, the use of this combination is contraindicated in pregnant women.
Since levocetirizine is excreted in breast milk, the use of this combination during breastfeeding is not recommended.
The ability to influence the reaction speed when driving or working with other mechanisms
In some clinical studies, cases of drowsiness, fatigue and asthenia have been reported in some patients treated with levocetirizine. Therefore, while taking levocetirizine, one should refrain from hazardous activities that require full attention and coordination of movements, namely working with mechanisms or driving vehicles.
Method of administration and doses
Adults and children over 15 years of age are prescribed 1 tablet once a day in the evening, regardless of meals. The tablets are swallowed whole, without chewing. The course of treatment is 14 days.
Children
The drug is used in children from 15 years of age.
Overdose
There are no data on overdose of this combination drug. However, cases of overdose with the individual substances have been reported.
Montelukast.
Acute overdose of montelukast has been reported in post-marketing experience and during clinical trials. The reports included doses exceeding 1000 mg in adults and children. The clinical and laboratory data were consistent with the safety profile for adults and children. No adverse events were observed in most of the overdose reports. The most commonly reported adverse events were consistent with the safety profile of montelukast and included abdominal pain, somnolence, thirst, headache, vomiting, and psychomotor hyperactivity. It is not known whether montelukast is removed by peritoneal dialysis or hemodialysis.
Treatment is symptomatic.
Levocetirizine hydrochloride.
Symptoms of overdose may include drowsiness in adults and agitation and anxiety in children, alternating with drowsiness. There is no specific antidote for levocetirizine. In the event of overdose, standard measures are recommended to remove unabsorbed drug. Gastric lavage may be performed shortly after ingestion. Hemodialysis is not effective in removing levocetirizine.
Treatment is symptomatic.
Adverse reactions
There are no data on the side effects of this combination drug. However, side effects of the individual substances have been reported.
Montelukast.
The most common side effects include dyspepsia, abdominal pain, rash, dizziness, headaches, fatigue, fever, injury, cough, nasal congestion, and flu.
Adverse reactions reported in the post-marketing period:
From the circulatory and lymphatic system: tendency to increased bleeding.
Immune system disorders: hypersensitivity reactions, including anaphylaxis, eosinophilic infiltration of the liver.
Psychiatric disorders: sleep disturbances, including nightmares, hallucinations, insomnia, irritability, anger, impatience, agitation, including aggressive behavior or hostility, tremor, depression, very rarely - suicidal thoughts and suicidal behavior.
Nervous system: lethargy and dizziness, paresthesia/hypoesthesia, seizures.
Respiratory, thoracic and mediastinal disorders: epistaxis.
Gastrointestinal: diarrhea, dry mouth, dyspepsia, nausea, vomiting.
Hepatobiliary: Elevations in serum transaminases (ALT, AST), rare cases of cholestatic hepatitis, hepatocellular and mixed hepatocellular disorders have been reported in patients receiving montelukast. Most of these cases were associated with other aggravating factors, such as concomitant medication or a history of liver disease, such as alcohol abuse or hepatitis.
Skin and subcutaneous tissue disorders: angioedema, hematoma, urticaria, pruritus, rash, erythema nodosum.
Musculoskeletal and connective tissue disorders: arthralgia, myalgia, including muscle cramps.
General disorders and local reactions: asthenia/fatigue, feeling of discomfort, swelling.
In isolated cases, Churg-Strauss syndrome (CSS) has been described in patients with asthma treated with montelukast. In isolated cases, patients with asthma receiving montelukast may experience systemic eosinophilia, sometimes with clinical manifestations of vasculitis, the so-called Churg-Strauss syndrome.
Levocetirizine hydrochloride.
The use of levocetirizine in patients aged 12 years and older has been associated with adverse reactions such as somnolence, fatigue, nasopharyngitis, dry mouth and pharyngitis. Uncommon adverse reactions have included asthenia and abdominal pain. Isolated cases of adverse reactions reported in the post-marketing period include:
Immune system disorders: hypersensitivity, including anaphylaxis.
On the part of the psyche: aggression, agitation, sleep disturbances, hallucinations, depression.
Nervous system: convulsions, fainting, tremor, vertigo, dysgeusia.
On the part of the organs of vision: visual impairment.
From the cardiovascular system: feeling of palpitations, tachycardia.
Respiratory, thoracic and mediastinal disorders: dyspnea.
Musculoskeletal and connective tissue disorders: myalgia.
Examination results: weight gain; deviation of liver function tests from normal values.
Gastrointestinal: nausea, diarrhea, vomiting, constipation, increased appetite, hepatitis.
Renal: dysuria, urinary retention.
Expiration date
2 years.
Storage conditions
Store in the original packaging at a temperature not exceeding 25ºС, out of the reach of children.
Packaging
Package No. 10 (10x1): 10 tablets in a blister, 1 blister in a cardboard box.
Packaging No. 20 (10x2): 10 tablets in a blister, 2 blisters in a cardboard box.
Vacation category
According to the recipe.
Producer
Bafna Pharmaceuticals Ltd., India.
Location of the manufacturer and address of its place of business
147, Madhavaram – Redhills Road Grantlyon Village Vadakarai Chennai Tamil Nadu IN 600052, India.
Applicant
SCAN BIOTECH LTD, India.
Applicant's location
E-4/300, Arera Colony Extension, 462016, Bhopal, (MP) India.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.