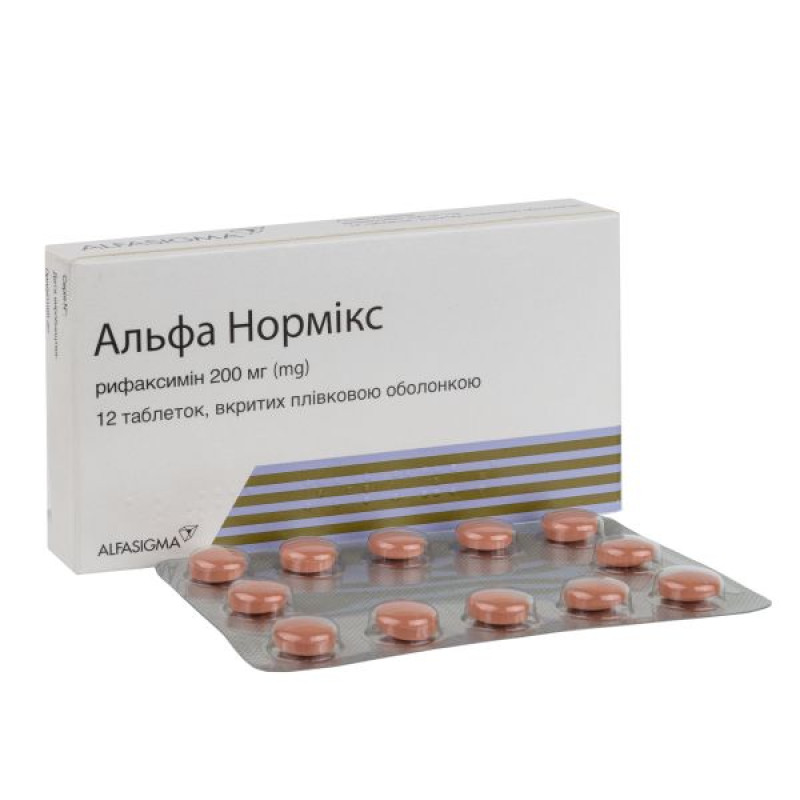
Alfa Normix film-coated tablets 200 mg blister No. 12

Instructions for use Alfa Normix film-coated tablets 200 mg blister No. 12
Composition
active ingredient: rifaximin;
1 film-coated tablet contains 200 mg of rifaximin;
excipients: sodium starch glycolate (type A), glycerol distearate, colloidal anhydrous silica, talc, microcrystalline cellulose, hypromellose, titanium dioxide (E 171), disodium edetate, propylene glycol, red iron oxide (E 172).
Dosage form
Film-coated tablets.
Main physicochemical properties: pink round biconvex tablets, film-coated.
Pharmacotherapeutic group
Drugs used in intestinal infections. Antibiotics. Rifaximin. ATC code A07A A11.
Pharmacological properties
Pharmacodynamics
The drug Alfa Normix contains the active substance rifaximin in the polymorphic form α. Rifaximin is a broad-spectrum antibiotic that is a semisynthetic derivative of rifamycin SV. Like other rifamycins, it irreversibly binds to the β-subunit of the bacterial DNA-dependent RNA polymerase and thus inhibits the synthesis of bacterial RNA and proteins. This irreversible binding to the enzyme determines the bactericidal effect of rifaximin against sensitive bacteria.
Rifaximin has a broad spectrum of antimicrobial activity against gram-positive and gram-negative aerobic and anaerobic bacteria that cause intestinal infections.
The broad spectrum of antibacterial action of rifaximin leads to a decrease in the number of pathogenic bacteria in the intestine that cause certain diseases or are involved in their pathogenesis.
Rifaximin may reduce:
production of ammonia and other toxic compounds by bacteria, which occurs during the pathogenesis and development of clinical symptoms of hepatic encephalopathy in severe liver diseases with disorders of detoxification function; hyperproliferation of bacteria in intestinal bacterial overgrowth syndrome; the number of bacteria in intestinal diverticula, which can cause intra-peridivertic inflammation and, presumably, significantly affect the development of symptoms and complications of diverticulosis; antigenic stimuli, which, in the presence of genetically determined defects in immunoregulation and/or barrier function of the intestinal mucosa, can provoke or maintain chronic intestinal inflammation; the risk of infectious complications during colorectal surgery.
Mechanism of resistance
The development of resistance to rifaximin is primarily due to reversible chromosomal single-step rearrangement of the rpoB gene, which encodes bacterial RNA polymerase. The prevalence of resistance among bacteria isolated from patients with traveler's diarrhea is very low.
Clinical studies examining changes in the susceptibility of intestinal flora in patients with traveler's diarrhea during a 3-day course of rifaximin treatment failed to identify drug-resistant Gram-positive (e.g., enterococci) or Gram-negative (E. coli) microorganisms.
The development of resistance in normal intestinal bacterial flora was studied after repeated high doses of rifaximin were administered to healthy volunteers and patients with inflammatory bowel disease. Rifaximin-resistant strains developed during the study, but they were unstable and did not colonize the gastrointestinal tract or displace rifaximin-sensitive strains. After cessation of treatment, the resistant strains disappeared rapidly.
Preclinical and clinical data suggest that treatment with rifaximin in patients carrying Mycobacterium tuberculosis and Neisseria meningitides will not result in the development of rifampicin resistance.
Sensitivity
Rifaximin is a non-absorbable antibacterial agent. In vitro susceptibility testing results cannot be used to reliably determine the susceptibility or resistance of bacteria to rifaximin. At this time, there are insufficient data to establish a clinical breakpoint for rifaximin.
The in vitro activity of rifaximin was evaluated against various pathogens causing travelers' diarrhea in 4 different regions of the world, namely ETEC (enterotoxigenic E. coli), EAEC (enteroaggregative E. coli), Salmonella spp., Shigella spp., Non-V. cholerae vibrios, Plesiomonas spp., Aeromonas spp., Campylobacter spp. The MIC90 (minimum inhibitory concentration) for the isolated bacterial samples, which was 32 μg/ml, is easily achieved in the intestinal lumen due to the high concentrations of rifaximin in feces.
Due to very low absorption from the gastrointestinal tract, rifaximin in the α polymorphic form acts locally in the intestinal lumen and is clinically ineffective against invasive pathogens, even if these bacteria are sensitive to it in vitro.
Clinical efficacy
Clinical trials in the treatment of patients with traveler's diarrhea have shown clinical efficacy of rifaximin against ETEC (enterotoxigenic E. coli) and EAEC (enteroaggregative E. coli). These bacteria are the main cause of traveler's diarrhea in people traveling to Mediterranean countries or tropical or subtropical regions.
Pediatric patient group
Both individual studies and meta-analyses of their results indicate a positive response to the use of rifaximin for the treatment of acute diarrhea when it is confirmed or suspected to be caused by non-invasive bacteria sensitive to rifaximin, such as E. coli.
In these limited clinical studies, the most commonly used dose for treating children aged 6 to 12 years was 20 to 30 mg rifaximin/kg/day, divided into 2 to 4 doses.
Pharmacokinetics
Absorption
Pharmacokinetic studies have shown that there is virtually no absorption of rifaximin in the α polymorphic form (less than 1%) after oral administration. Comparative pharmacokinetic studies have shown that the absorption of other polymorphic forms of rifaximin is greater than that of the α polymorphic form.
After administration of therapeutic doses of the drug in both healthy volunteers and patients with damaged intestinal mucosa (patients with ulcerative colitis or Crohn's disease), rifaximin plasma levels were very low (less than 10 ng/ml).
When rifaximin was administered within 30 minutes of a high-fat meal, a clinically insignificant increase in systemic absorption of rifaximin was observed.
Distribution
Rifaximin is moderately bound to human plasma proteins. In vivo, the mean protein binding of rifaximin was 67.5% in healthy volunteers and 62% in patients with liver disease.
Metabolism
Studies have shown that rifaximin is not metabolized during passage through the gastrointestinal tract.
It has been found that 0.025% of the administered dose of rifaximin is excreted in the urine and less than 0.01% of the dose is metabolized to 25-desacetylrifaximin, the only identified metabolite of rifaximin in humans.
Breeding
The results of a study with radiolabeled rifaximin suggest that it is almost exclusively and completely excreted in the feces (96.9% of the administered dose). Urinary excretion of labeled rifaximin does not exceed 0.4% of the administered dose.
Linearity/nonlinearity
The rate and extent of systemic exposure of rifaximin in humans are believed to be characterized by nonlinear dose-dependent kinetics, consistent with the possibility that absorption may be limited by the dissolution rate of rifaximin.
Pediatric patient group
The pharmacokinetic parameters of rifaximin have not been studied in pediatric patients of any age.
Indication
Gastrointestinal infections caused by bacteria sensitive to rifaximin, such as acute gastrointestinal infections and travelers' diarrhea; small intestinal bacterial overgrowth syndrome; hepatic encephalopathy; diverticular disease of the intestine (diverticulitis) in the acute stage and chronic inflammation of the intestine; prevention of infectious complications in colorectal surgery.
Contraindication
Hypersensitivity to rifaximin, other rifamycin derivatives or to any of the excipients; hypersensitivity reactions include exfoliative dermatitis, angioedema and anaphylaxis; intestinal obstruction; severe ulcerative lesions of the intestine.
Interaction with other medicinal products and other types of interactions
There is no experience with the simultaneous use of rifaximin with other antibacterial agents of the rifamycin group for the treatment of systemic bacterial infections.
In vitro data indicate that rifaximin does not inhibit the major cytochrome P450 enzymes (1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1 and 3A4) involved in drug metabolism. In in vitro induction studies, rifaximin did not induce CYP1A2 and CYP2B6, but was a weak inducer of the cytochrome P450 isoenzyme CYP3A4.
In clinical drug interaction studies in healthy volunteers, rifaximin has not been shown to significantly affect the pharmacokinetics of CYP3A4 substrates. However, in patients with hepatic impairment, it cannot be excluded that rifaximin may reduce the efficacy of concomitantly administered CYP3A4 substrates (e.g. warfarin, antiepileptic and antiarrhythmic drugs, and oral contraceptives) due to increased systemic exposure to rifaximin in these patients compared to healthy volunteers.
Both decreases and increases in international normalized ratio have been reported when rifaximin is administered to patients taking warfarin. If such co-administration is necessary, careful monitoring of the international normalized ratio should be performed when rifaximin is added or withdrawn. Dosage adjustments of oral anticoagulants may be necessary.
In vitro studies suggest that rifaximin is a moderate affinity substrate for P-glycoprotein (P-gp) and that it is metabolized by CYP3A4. It is unknown whether concomitant use with drugs that inhibit CYP3A4 may increase the systemic exposure of rifaximin.
In vitro studies have examined the potential for drug interactions occurring at the level of transport systems; the results of these studies suggest that clinical interactions between rifaximin and other compounds that are excreted by P-gp and other transport proteins (MRP2, MRP4, BCRP and BSEP) are unlikely.
In case of concomitant use, rifaximin should be taken at least 2 hours after taking activated charcoal.
Application features
Clinical data indicate that rifaximin is ineffective in the treatment of intestinal infections caused by invasive enteric pathogens such as Campylobacter jejuni, Salmonella spp. and Shigella spp., which usually cause diarrhea accompanied by fever, bloody and very frequent stools. If the symptoms of diarrhea worsen or do not improve within 48 hours, the drug should be discontinued and alternative antimicrobial therapy should be prescribed.
Clostridium difficile-associated diarrhea (CDAD) has been reported with nearly all antibiotics, including rifaximin. A potential association of rifaximin treatment with CDAD and pseudomembranous colitis cannot be excluded.
Rifaximin should be administered with caution concomitantly with P-glycoprotein inhibitors such as cyclosporine.
Despite its low absorption, rifaximin, like other rifamycin derivatives, can stain urine reddish, which patients should be warned about.
Both decreases and increases in international normalized ratio (associated with bleeding events in some cases) have been reported when rifaximin is administered to patients taking warfarin. If such co-administration is necessary, careful monitoring of the international normalized ratio should be performed when rifaximin is added or withdrawn. Dosage adjustments of oral anticoagulants may be necessary to maintain the desired level of anticoagulation.
Ability to influence reaction speed when driving vehicles or other mechanisms
If dizziness or drowsiness occurs during treatment with the drug, you should refrain from driving vehicles and operating other mechanisms.
Use during pregnancy or breastfeeding
There are no data on the use of rifaximin in pregnant women. Animal studies have not shown direct or indirect harmful effects of rifaximin on fertility.
As a precautionary measure, the use of rifaximin during pregnancy is not recommended.
It is not known whether rifaximin and its metabolites are excreted in human milk, therefore a risk to the breastfed infant cannot be excluded. Therefore, a decision should be made whether to discontinue breast-feeding or to discontinue/abstain from the drug during breast-feeding, taking into account the benefit of breast-feeding for the child and the need for therapy for the mother.
Method of administration and doses
Adults and children over 12 years of age: from 1 tablet 3 times a day to 2 tablets 2-3 times a day (corresponds to a daily dose of 600-1200 mg of rifaximin).
The duration of treatment should not exceed 7 days and depends on the clinical response to treatment. A repeated course of treatment can be carried out with a break of 20-40 days.
The doses used and the frequency of administration may be changed on the recommendation of a doctor.
Method of application
Take orally with a glass of water. The drug can be taken regardless of meals.
Certain patient groups
Elderly patients
Since there are no differences in the safety and efficacy of rifaximin when used in young and elderly patients, no dose adjustment is necessary when prescribing the drug to elderly patients.
Patients with liver damage
Available clinical data indicate an increase in systemic exposure to rifaximin in patients with hepatic impairment compared with healthy volunteers. However, the increase in systemic exposure to rifaximin in patients with hepatic impairment should be considered in light of the local effects of rifaximin in the gastrointestinal tract and its low systemic bioavailability, as well as the available safety data on the use of rifaximin in patients with cirrhosis. Therefore, due to the local effects, dose adjustment of rifaximin is not recommended in such patients.
Patients with kidney damage
There are no clinical data on the use of rifaximin in patients with renal impairment. Although no dose adjustment is expected for such patients, caution should be exercised when prescribing rifaximin to patients with renal impairment.
Children
Since the efficacy, dosage, and safety of rifaximin in the treatment of children under 12 years of age have not been established, there are no dosage recommendations for these patients.
Overdose
In clinical trials in patients with traveler's diarrhea, doses of rifaximin up to 1800 mg/day were tolerated without any severe clinical manifestations. Doses of up to 2400 mg rifaximin per day for 7 days in patients and healthy volunteers did not result in any significant clinical symptoms associated with the use of high doses.
In case of overdose, symptomatic and supportive treatment is recommended.
Adverse reactions
Clinical studies: Double-blind controlled trials and clinical pharmacology studies comparing rifaximin with placebo or other antibiotics have yielded results that allow for a quantitative assessment of the incidence of adverse reactions.
Note: Most of the listed adverse reactions (especially gastrointestinal disorders) may also be symptoms of the underlying disease; in clinical trials they were observed with the same frequency as with placebo.
Post-marketing experience: During post-marketing studies with rifaximin, the following adverse reactions were additionally reported, the frequency of which is unknown (cannot be estimated from the available data).
Adverse reactions, the development of which is at least possibly related to rifaximin, are classified according to the system organ classes, as well as the frequency of occurrence as follows: very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥ 1/1000 to < 1/100); rare (< 1/10000); frequency unknown (frequency cannot be estimated from the available data).
Infections and infestations
Uncommon: candidiasis, herpes infection, nasopharyngitis, pharyngitis, upper respiratory tract infection.
Frequency unknown: Clostridial infection.
Blood and lymphatic system disorders
Uncommon: lymphocytosis, monocytosis, neutropenia.
Frequency unknown: thrombocytopenia.
Immune system disorders
Frequency unknown: anaphylactic reactions, hypersensitivity reactions.
Metabolic and nutritional disorders
Uncommon: decreased appetite, dehydration.
Mental disorders
Uncommon: abnormal sleep, depressed mood, insomnia, nervousness.
Nervous system disorders
Common: dizziness, headache.
Uncommon: hypoaesthesia, migraine, paraesthesia, sinus headache, drowsiness.
Frequency unknown: presyncope.
Disorders of the organ of vision
Uncommon: diplopia.
Hearing and inner ear disorders
Uncommon: ear pain, vertigo.
Heart disorders
Uncommon: rapid heartbeat.
Vascular disorders
Uncommon: increased blood pressure, hot flashes.
Respiratory, thoracic and mediastinal disorders
Uncommon: cough, dry throat, dyspnea, nasal congestion, oropharyngeal pain, rhinorrhea.
Gastrointestinal disorders
Common: abdominal pain, constipation, sudden urge to defecate, diarrhea, flatulence, abdominal distension, nausea, vomiting, rectal tenesmus.
Uncommon: upper abdominal pain, ascites, dry lips, dyspepsia, gastrointestinal motility disorders, hard stools, bloody or mucous stools, taste disturbances.
Hepatobiliary disorders
Uncommon: increased aspartate aminotransferase.
Frequency unknown: Abnormal liver function tests.
Skin and subcutaneous tissue disorders
Uncommon: rash, urticaria and exanthema, sunburn (not photosensitivity, but sunburn).
Frequency unknown: angioedema, exfoliative dermatitis, dermatitis, eczema, erythema, pruritus, purpura, urticaria.
Musculoskeletal and connective tissue disorders
Uncommon: back pain, muscle spasm, muscle weakness, myalgia, neck pain.
Kidney and urinary tract disorders
Uncommon: hematuria, glycosuria, pollakiuria, polyuria, proteinuria.
Reproductive system and mammary gland disorders
Uncommon: polymenorrhea.
General disorders and administration site conditions
Common: fever.
Uncommon: asthenic conditions, chills, cold sweat, hyperhidrosis, flu-like syndrome, peripheral edema, pain and discomfort.
Others
Frequency unknown: deviation of international normalized ratio values.
Expiration date
3 years.
Do not use after the expiry date stated on the packaging.
Storage conditions
Does not require any special storage conditions. Keep out of the reach of children.
Packaging
12 tablets in a blister; 1 blister in a cardboard box.
Vacation category
According to the recipe.
Producer
Alfasigma S.p.A./Alfasigma SpA
Location of the manufacturer and its business address
Via Enrico Fermi 1, 65020 Alanno (Pescara), Italy.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.