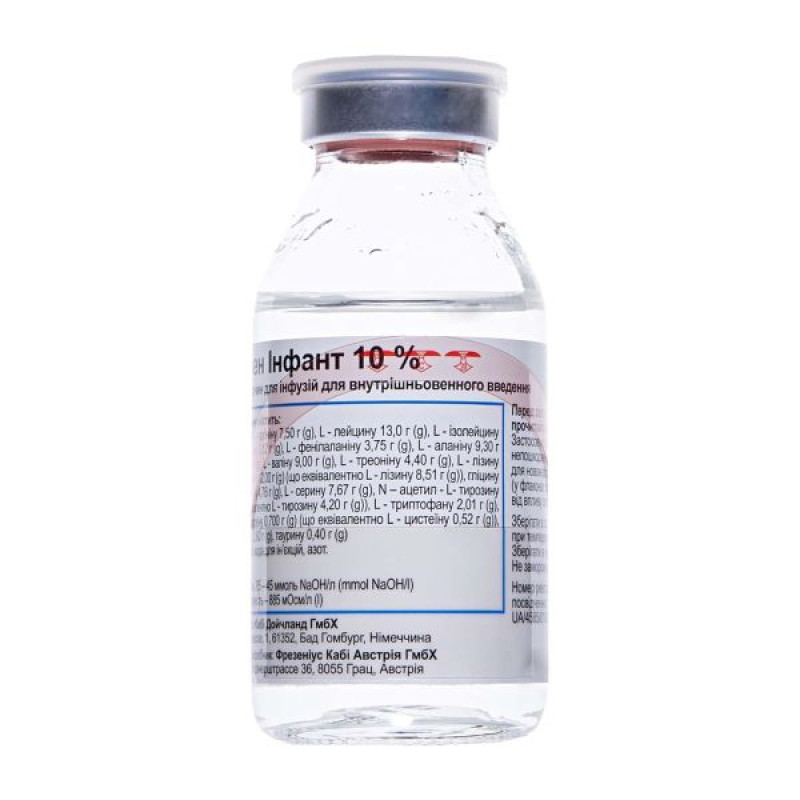
Aminoven Infant 10% solution for infusions 10% bottle 100 ml
Pharmacological properties
Pharmacodynamics. The amino acids that make up the drug Aminoven Infant 10% are physiological components. After parenteral administration, they are included in the pool of free amino acids of the body and participate in all metabolic processes, in particular, they are used for protein synthesis.
Pharmacokinetics. The bioavailability of the drug Aminoven Infant 10% when administered intravenously is 100%. Amino acids are included in the total pool of free amino acids of the body and are distributed in the interstitial fluid and intercellular space of organs and tissues. The concentration of free amino acids in the blood plasma and cytoplasm of cells is regulated within narrow limits depending on the age, nature of nutrition and the general condition of the patient. When administered correctly (slowly and at a constant rate), Aminoven Infant 10% does not disrupt the balance of amino acids. In severe liver and kidney dysfunction, the regulation of the balance of amino acids is disrupted. In these cases, other compositions of amino acid solutions should be used for parenteral nutrition. Only a small part of the amino acids is eliminated by the kidneys. T ½ of amino acids from blood plasma depends largely on age.
Indication
Aminoven Infant 10% is intended for partial parenteral nutrition of premature infants, newborns and young children. Compatible with carbohydrate solutions, fat emulsions, as well as preparations of vitamins, electrolytes and trace elements, it provides complete parenteral nutrition.
Application
Aminoven Infant 10% is intended for long-term intravenous drip administration, preferably into central veins.
Maximum rate of administration: 0.1 g of amino acids per 1 kg of body weight per hour, which is equal to 1 ml/kg of body weight per hour.
Maximum daily dose:
children under 1 year old - 1.5-2.5 g of amino acids per 1 kg of body weight per day or 15-25 ml of Aminoven Infant 10% solution per 1 kg of body weight per day; children aged 2-5 years old - 1.5 g of amino acids per 1 kg of body weight per day or 15 ml/kg of body weight per day; children aged 6-14 years old - 1.0 g of amino acids per 1 kg of body weight per day or 10 ml/kg of body weight per day.Aminoven Infant 10% is used as long as the need for parenteral nutrition persists.
Contraindication
Disturbances of amino acid metabolism, metabolic acidosis, hyperhydration, hyperkalemia, hypersensitivity to the drug, shock, hypoxia, decompensated heart failure, renal failure in the absence of hemodialysis or hemofiltration, severe hepatic failure. Individual dosage is required for hepatic and renal failure. Use with caution in patients with hyponatremia.
Side effects
When used correctly, no allergic reactions have been observed. When administering Aminoven Infant 10% into peripheral veins, local reactions may occur: hyperemia, phlebitis, thrombosis. Daily monitoring of the puncture site is recommended.
Special instructions
When parenteral nutrition of young children, the following indicators must be taken into account: urine nitrogen, ammonia content, glucose, electrolytes, triglycerides (with additional administration of fat emulsions), liver enzymes, serum osmolarity, acid-base balance, and water-salt metabolism.
Too rapid an infusion can lead to loss of amino acids through the kidneys and consequently to an amino acid imbalance.
Incompatibility: Due to the increased risk of microbial contamination, amino acid solutions should not be mixed with other drugs.
The addition of other drugs to Aminoven Infant 10% should be avoided as this may cause toxic reactions. In any case, ensure compatibility of the drugs, maintain sterility and mix thoroughly. Solutions with the addition of other drugs should not be stored.
Children. Aminoven Infant 10% is intended for partial parenteral nutrition of premature infants, newborns and young children.
Effects on the ability to drive or use machines. Not studied.
Interactions
Currently, there are no known cases of interaction of Aminoven Infant 10% with other medicines.
Overdose
If the dosage or rate of administration of the drug Aminoven Infant 10% is exceeded, as well as with an overdose of other amino acid solutions, chills, nausea, vomiting, renal aminoacidosis occur. In this case, the administration of the drug should be stopped immediately. If hyperkalemia occurs, inject from 200 to 500 ml of 5% glucose solution (glucose) with the addition of 1-3 IU of insulin for every 3-5 g of glucose (glucose). It is possible to continue therapy with the drug using low doses.
Storage conditions
In a place protected from light at a temperature not exceeding 25 °C. Do not freeze. Shelf life - 2 years.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.