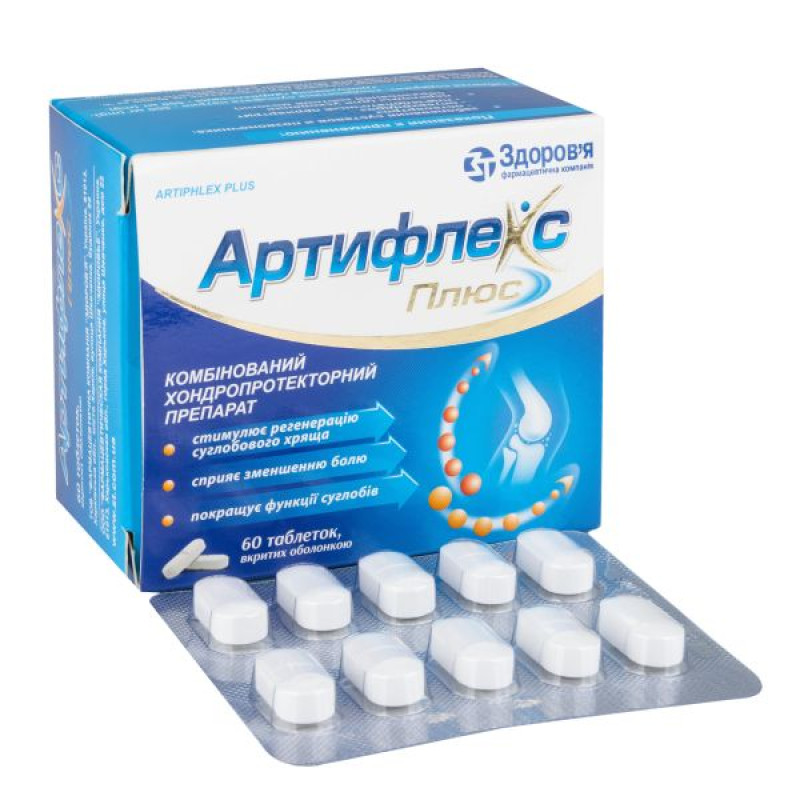
Artiflex Plus film-coated tablets 1000 mg blister No. 60

Instructions Artiflex Plus film-coated tablets 1000 mg blister No. 60
Composition
active ingredients: 1 tablet contains chondroitin sodium sulfate 500 mg, glucosamine hydrochloride 500 mg;
excipients: crospovidone, microcrystalline cellulose, croscarmellose sodium, magnesium stearate, calcium hydrogen phosphate, colloidal anhydrous silicon dioxide, dry mixture "Opadry II white", containing: titanium dioxide (E 171), talc, polyethylene glycol (macrogol), polyvinyl alcohol.
Dosage form
Film-coated tablets.
Main physicochemical properties: film-coated tablets, white or almost white in color, elongated in shape, with a biconvex surface, with a score.
Pharmacotherapeutic group
Nonsteroidal anti-inflammatory and antirheumatic drugs.
ATX code M01A X.
Pharmacological properties
Pharmacodynamics
A combined chondroprotective drug, the pharmacological action of which is due to the components included in its composition.
Chondroitin sulfate is a high-molecular mucopolysaccharide that participates in the construction of cartilage tissue. Reduces the activity of enzymes that destroy articular cartilage and stimulates the regeneration of articular cartilage. Chondroitin sulfate reduces the activity of the inflammatory process in the early stages and, thus, slows down the degeneration of cartilage tissue. Helps reduce pain, improve joint function, reduces the need for non-steroidal anti-inflammatory drugs in patients with osteoarthritis of the knee and hip joints.
Glucosamine hydrochloride has chondroprotective properties, reduces glucosamine deficiency in the body, participates in the biosynthesis of proteoglycans and hyaluronic acid. Glucosamine hydrochloride initiates the process of sulfur fixation during the synthesis of chondroitin sulfate acid. It selectively acts on articular cartilage, is a specific substrate and stimulator of the synthesis of hyaluronic acid and proteoglycans, inhibits the formation of superoxide radicals and enzymes that cause damage to cartilage tissue (collagenase and phospholipase), prevents the destructive effect of glucocorticoids on chondrocytes and disruption of the biosynthesis of glycosaminoglycans caused by nonsteroidal anti-inflammatory drugs.
Indication
Degenerative-dystrophic diseases of the joints and spine: osteoarthritis, shoulder-scapular periarthritis, osteochondrosis; fractures (to accelerate the formation of bone callus).
Contraindication
Hypersensitivity to any component of the drug. Phenylketonuria, bleeding tendency, thrombophlebitis, decompensated liver or kidney dysfunction, diabetes mellitus. Do not use the drug if you are allergic to shellfish.
Interaction with other medicinal products and other types of interactions
Chondroitin sulfate may enhance the effect of anticoagulants, which requires more frequent monitoring of blood clotting parameters during simultaneous use.
When taken simultaneously, glucosamine enhances the absorption of tetracycline antibiotics in the digestive tract and reduces the absorption of penicillins and chloramphenicol. It shows synergistic action when used simultaneously with chondroitin and other chondroprotectors. There are reports that glucosamine can affect the blood concentration of cyclosporine and warfarin.
When used simultaneously with glucocorticosteroids and nonsteroidal anti-inflammatory drugs, glucosamine and chondroitin may reduce the need for them, as well as for painkillers.
The effectiveness of treatment increases when the diet is enriched with vitamins A, C and salts of manganese, magnesium, copper, zinc, and selenium.
Application features
Do not exceed the recommended dose.
Ability to influence reaction speed when driving vehicles or other mechanisms
Studies on the effect of the drug on the ability to drive a car or other (potentially dangerous) mechanisms have not been conducted. However, side effects from the nervous system that occur during the use of the drug (drowsiness, dizziness) should be taken into account.
Use during pregnancy or breastfeeding
The drug is contraindicated during pregnancy. Breastfeeding should be discontinued during treatment with the drug.
Method of administration and doses
Take orally with water.
Adults are prescribed 1 tablet orally 1-3 times a day. It is recommended to start treatment with a higher dose - 1 tablet 3 times a day, gradually switching to a maintenance dose. The minimum duration of the course of treatment is 6 weeks; the clinical effect is observed after 2-3 months of using the drug. The clinical effect occurs slowly and persists for a long time after stopping the drug.
Children
The drug should not be used in children.
Overdose
In case of overdose, side effects may be increased. In case of accidental acute overdose, vomiting should be induced. Further treatment is symptomatic.
Adverse reactions
On the part of the digestive tract: dyspeptic disorders, nausea, vomiting, stomach pain, constipation, diarrhea, flatulence.
From the nervous system: drowsiness, headache, dizziness, general weakness, insomnia, increased fatigue.
Others: hair loss, vision disorders, general weakness, swelling.
Expiration date
3 years.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 °C.
Keep out of reach of children.
Packaging
10 tablets in a blister; 6 blisters in a cardboard box.
Vacation category
Without a prescription.
Producer
Limited Liability Company "Pharmaceutical Company "Zdorovya".
Location of the manufacturer and its business address
Ukraine, 61013, Kharkiv region, Kharkiv city, Shevchenko street, building 22.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.