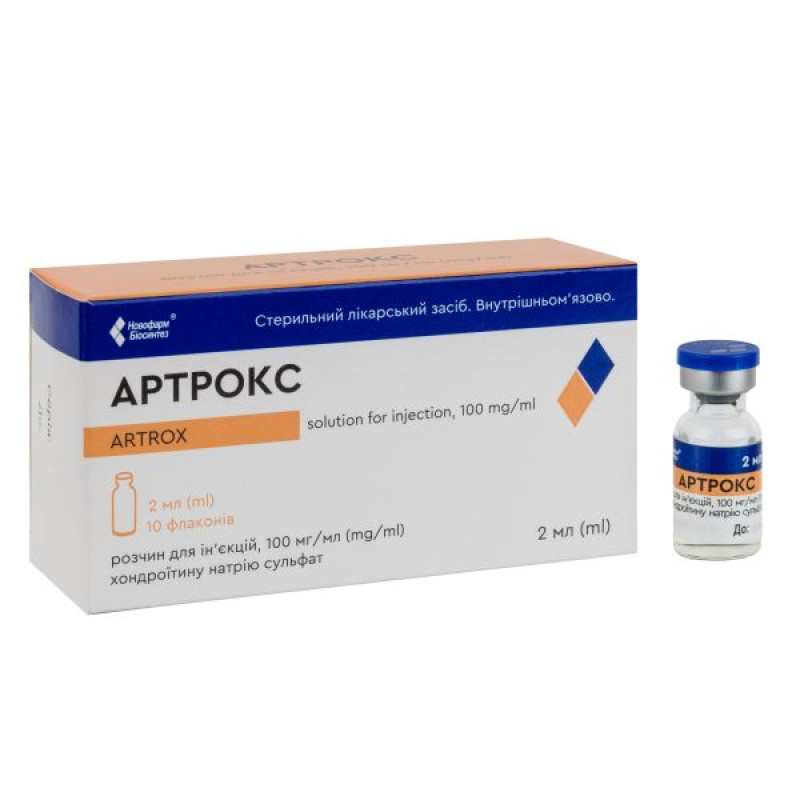
Artrox solution for injection 100 mg/ml bottle 2 ml No. 10

Instructions Artrox solution for injection 100 mg/ml bottle 2 ml No. 10
Composition
active ingredient: chondroitin sulfate sodium;
1 vial (2 ml of the drug) contains chondroitin sodium sulfate 200 mg;
Excipients: benzyl alcohol, water for injections.
Dosage form
Solution for injection.
Main physicochemical properties: transparent colorless or slightly yellowish solution.
Pharmacotherapeutic group
Drugs affecting the musculoskeletal system. Chondroitin sulfate.
ATX code M01A X25.
Pharmacological properties
Pharmacodynamics
The main active ingredients of the drug are sodium salts of chondroitin sulfate A and C (average molecular weight 11,000 daltons). Chondroitin sulfate is a high-molecular mucopolysaccharide. It is the main component of proteoglycans, which together with collagen fibers form the cartilage matrix.
The drug inhibits the process of degeneration and stimulates the regeneration of cartilage tissue, has a chondroprotective, anti-inflammatory, analgesic effect. Replaces chondroitin sulfate of articular cartilage, catabolized by the pathological process. Inhibits the activity of enzymes that cause degradation of articular cartilage: inhibits metalloproteinases, namely leukocyte elastase. Reduces the activity of hyaluronidase. Partially blocks the release of free oxygen radicals; helps block chemotaxis, antigenic determinants. Stimulates the production of proteoglycans by chondrocytes. Affects phosphorus-calcium metabolism in cartilage tissue. Allows you to restore the mechanical and elastic integrity of the cartilage matrix. Anti-inflammatory and analgesic effects are achieved by reducing the release of inflammatory mediators and pain factors into the synovial fluid by synoviocytes and macrophages of the synovial membrane, as well as by inhibiting the secretion of leukotriene B and prostaglandin E.
The use of the drug promotes the restoration of the joint capsule and cartilaginous surfaces of the joints, prevents compression of connective tissue, promotes lubrication of the joint surfaces, normalizes the production of joint fluid, improves joint mobility, helps reduce the intensity of pain, and improves the quality of life.
Artrox slows down bone resorption and reduces calcium loss, accelerating bone tissue repair processes.
Pharmacokinetics
After intramuscular injection, chondroitin sulfate penetrates into the synovial fluid. The maximum concentration in the synovial fluid is reached 4-5 hours after injection. It is excreted from the body within 24 hours. It is eliminated mainly by the kidneys.
Clinical characteristics.
Indication
Degenerative-dystrophic diseases of the joints and spine (primary arthrosis, intervertebral osteochondrosis, osteoarthritis), osteoporosis, periodontal diseases, fractures (to accelerate the formation of bone callus). Treatment of the consequences of joint operations.
Contraindication
Increased individual sensitivity to any of the components of the drug, tendency to bleeding, thrombophlebitis, renal failure, pregnancy, breastfeeding.
Interaction with other medicinal products and other types of interactions
When used simultaneously with chondroitin sulfate, it can reduce the need for glucocorticosteroids and nonsteroidal anti-inflammatory drugs, as well as analgesics. It shows synergistic action when used simultaneously with glucosamine and other chondroprotectors. The effectiveness of treatment increases when the diet is enriched with vitamins A, C and salts of manganese, magnesium, copper, zinc and selenium.
When taken simultaneously with acetylsalicylic acid or other anticoagulants or antiplatelet agents, it is recommended to monitor blood clotting.
Application features
To achieve a stable clinical effect, at least 25 injections of the drug are required. The effect persists for many months after the end of treatment. Repeated courses of treatment are used to prevent exacerbations. It is recommended to increase doses under the supervision of a physician for patients with excess body weight, peptic ulcer of the stomach or duodenum, while taking diuretics, as well as at the beginning of treatment if there is a need to accelerate the clinical response.
In case of allergic reactions or hemorrhages, treatment should be discontinued.
Use during pregnancy or breastfeeding
Use during pregnancy and breastfeeding is contraindicated.
Ability to influence reaction speed when driving vehicles or other mechanisms
There are no restrictions on driving vehicles and complex mechanisms when using the drug.
Method of administration and doses
The drug is administered intramuscularly to adults in a dose of 1 ml every other day. In case of good tolerance, the dose is increased to 2 ml, starting from the fourth injection. The course of treatment is 25-35 injections. Repeated courses are given after 6 months.
Children
There is no experience with the use of the drug in children.
Overdose
To date, no cases of overdose with the drug Artrox have been reported. However, it can be assumed that if the daily dose is exceeded, the side effects of the drug may be increased. Treatment is symptomatic.
Adverse reactions
When using the drug in people with hypersensitivity to the drug, the following disorders are possible:
from the immune system: allergic reactions, anaphylactic shock, angioedema;
Skin and subcutaneous tissue disorders: skin rash, itching, erythema, urticaria, dermatitis, alopecia.
Redness and itching may occur at the injection site.
Gastrointestinal disorders are possible: nausea, vomiting, abdominal pain, flatulence, dyspeptic phenomena.
Others: visual disturbances, keratopathy, dizziness, peripheral edema.
Expiration date
2 years.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 ° C. Keep out of the reach of children. Do not use any remaining medicine.
Packaging
2 ml in a vial; 5 vials in a contour blister pack; 2 contour blister packs in a cardboard pack.
Vacation category
According to the recipe.
Producer
LLC "Novopharm-Biosyntez".
Location of the manufacturer and its business address
Ukraine, 11700, Zhytomyr region, Novograd-Volynskyi, Zhytomyrska st., 38.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.