ATF-Long tablets 20 mg blister No. 40
Instructions for use ATF-Long tablets 20 mg blister No. 40
Composition
active ingredients: 1 tablet contains ATP-LONG (calculated on a substance that does not contain sodium chloride and water) – 20 mg with a content of 12.6 mg of adenine nucleotides;
excipients: powdered sugar; lactose monohydrate; magnesium stearate; colloidal anhydrous silicon dioxide.
Dosage form
Pills.
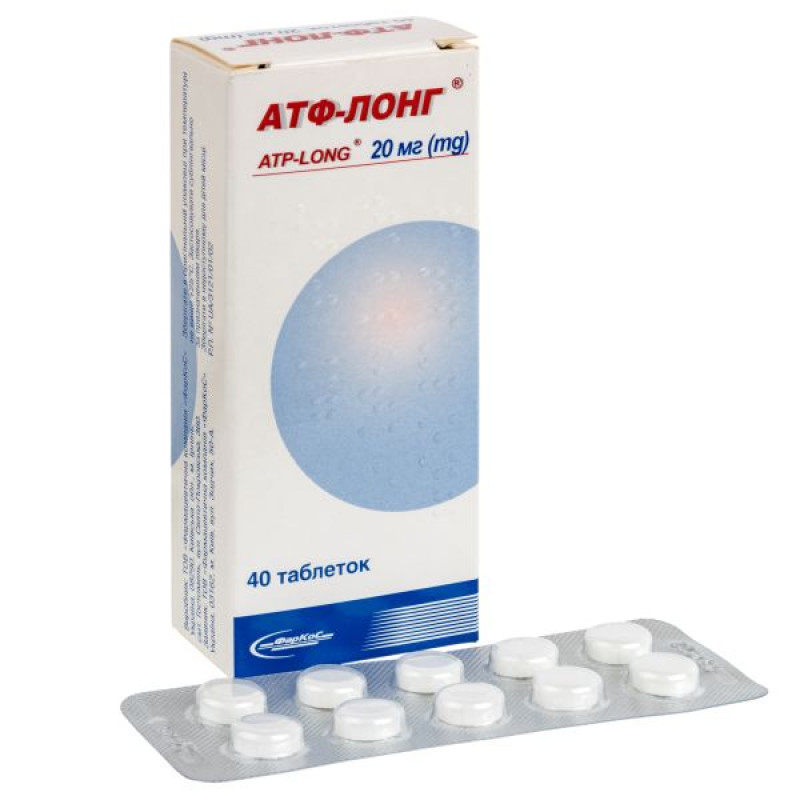
Main physicochemical properties: 20 mg tablets, white in color with a flat surface, a bevel and a score.
Pharmacotherapeutic group
Cardiological drugs. ATX code C01E B10.
Pharmacological properties
Pharmacodynamics.
ATP-LONG® is a drug of a new class of substances - multi-ligand coordination compounds with macroergic phosphates, the molecule of which consists of adenosine-5'-triphosphate (ATP), the amino acid histidine, magnesium and potassium salts and has a pharmacological effect characteristic only for it, which is not inherent to any individual chemical component (ATP, histidine, K+, Mg+ +).
ATP-LONG® affects metabolic processes in the myocardium, normalizes energy metabolism, the activity of ion transport systems of cell membranes, indicators of the lipid composition of membranes, the activity of membrane-bound enzymes, improves the antioxidant system of myocardial protection, has an anti-ischemic, membrane-stabilizing effect, and an antiarrhythmic effect in supraventricular tachycardia.
The drug has an energy-saving effect by inhibiting the activity of the enzyme 5'-nucleotidase, which is responsible for the rate of hydrolysis of energy substrates. ATP-LONG® prevents structural and functional damage to the plasma membranes of cardiomyocytes, ensuring the preservation of the quantitative and qualitative composition of membrane lipids, inhibiting the activity of membrane-bound phospholipases. ATP-LONG® inhibits the intensity of lipid peroxidation (LPO), thereby reducing the accumulation in the membranes of products of hydrolysis and peroxidation of phospholipids of fatty acids, lysophospholipids, which are characterized by pronounced detergent properties and the ability to cause disturbances in the contractile and rhythmic activity of the heart during ischemia. In the case of experimental myocardial ischemia, the drug increases the activity of Na + - K +- ATPase and Ca2+, Mg2+ - ATPase and the calcium-binding potential of the membrane.
ATP-LONG® improves central and peripheral hemodynamics, coronary circulation, normalizes myocardial contractility, which leads to an increase in physical performance. In conditions of ischemia, the drug reduces myocardial oxygen consumption, activates the functional state of the heart, increases cardiac output, which leads to a decrease in the frequency of angina attacks and shortness of breath during physical exertion.
ATP-LONG® restores normal sinus rhythm in patients with paroxysmal supraventricular tachycardia, with atrial fibrillation and flutter, and also reduces the activity of ectopic rhythm sources (atrial and ventricular extrasystoles).
ATP-LONG® normalizes the concentration of potassium and magnesium in tissues and reduces the concentration of uric acid.
Pharmacokinetics.
When administered into the body, ATP-LONG® slowly breaks down to form adenosine.
Indication
Complex therapy:
coronary heart disease, unstable angina, angina at rest and exertion;
post-infarction and myocardial cardiosclerosis (diffuse and focal cardiosclerosis);
heart rhythm disorders;
neurocirculatory dystonia;
myocardial dystrophies;
myocarditis;
chronic fatigue syndrome.
Contraindication
Hypersensitivity to the drug and its components;
acute myocardial infarction;
cardiogenic and other types of shock;
obstructive diseases of the bronchopulmonary system;
severe forms of bronchial asthma;
sinoatrial block;
atrioventricular block II-III degree;
hyperkalemia, hypermagnesemia;
hemorrhagic stroke.
Interaction with other medicinal products and other types of interactions
ATP-LONG® should not be used simultaneously with cardiac glycosides due to the increased risk of atrioventricular block.
When taken simultaneously with potassium-sparing diuretics, potassium preparations and ACE inhibitors, the risk of developing hyperkalemia increases, and with Magnerot and other magnesium preparations - hypermagnesemia.
Dipyridamole enhances the therapeutic effect of ATP-LONG®, while xanthinol nicotinate, caffeine, theophylline, and aminophylline reduce it.
ATP-LONG® may enhance the antianginal effect of alpha- and beta-blockers, calcium channel blockers, and nitrates.
Application features
Use with caution in severe arterial hypotension; simultaneously with cardiac glycosides due to increased risk of atrioventricular block, with a tendency to bronchospasm.
With prolonged use, it is necessary to monitor the level of potassium and magnesium in the blood.
The preparation contains powdered sugar and lactose, so patients with diabetes should use the preparation with caution. Patients with hereditary intolerance to glucose-galactose, sucrose-isomaltose should not use the preparation. Limit the use of products containing caffeine (coffee, tea, cola drinks).
Use during pregnancy or breastfeeding
There are no clinical data on the safety and efficacy of the drug during pregnancy, therefore its use in pregnant women is contraindicated. Breastfeeding should be discontinued during treatment.
Ability to influence reaction speed when driving vehicles or other mechanisms
If dizziness or low blood pressure occurs during treatment with the drug, you should refrain from driving vehicles and working with other mechanisms.
Method of administration and doses
ATF-LONG® tablets should be taken under the tongue (sublingually) and held until completely dissolved. Single dose – 10*-40 mg 3-4 times a day, regardless of meals.
In acute cardiac conditions (angina attack, arrhythmia), ATF-LONG® tablets should be taken at 10*-80 mg until the condition improves. The maximum daily dose is 160 mg.
The duration of treatment is determined by the doctor, on average it is 20-30 days. If necessary, repeat the course after 10-15 days. (* apply in the appropriate dosage)
Children
Do not use on children.
Overdose
Symptoms: possible development of bradycardia, atrioventricular block, arterial hypotension, syncope associated with a sudden decrease in blood pressure.
Treatment: discontinue the drug and begin symptomatic therapy. In case of bradycardia, administer atropine sulfate.
Adverse reactions
Skin and subcutaneous tissue disorders: skin rash, itching, facial flushing, angioedema.
Respiratory system: bronchospasm.
From the cardiovascular system: decreased blood pressure, tachycardia, atrioventricular block.
Gastrointestinal: nausea, feeling of discomfort in the epigastric region, increased motility of the digestive tract.
From the urinary system: increased diuresis.
From the nervous system: headache, dizziness.
Vascular: feeling of heat.
On the part of the immune system: allergic reactions, including skin rashes, itching, angioedema.
Expiration date
3 years.
The drug should not be used after the expiration date indicated on the package.
Storage conditions
Store in original packaging at a temperature not exceeding 5 °C.
Keep out of reach of children.
Packaging
10 tablets in a blister; 3 or 4 blisters in a box with labeling in Ukrainian and Russian.
Vacation category
Without a prescription.
Producer
LLC "Pharmaceutical Company "FarKoS".
Location of the manufacturer and its business address
08290, Ukraine, Kyiv region, Irpin city, Gostomel town, Lenina st., 360.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.