Brinera suspension eye drops 5ml No. 1
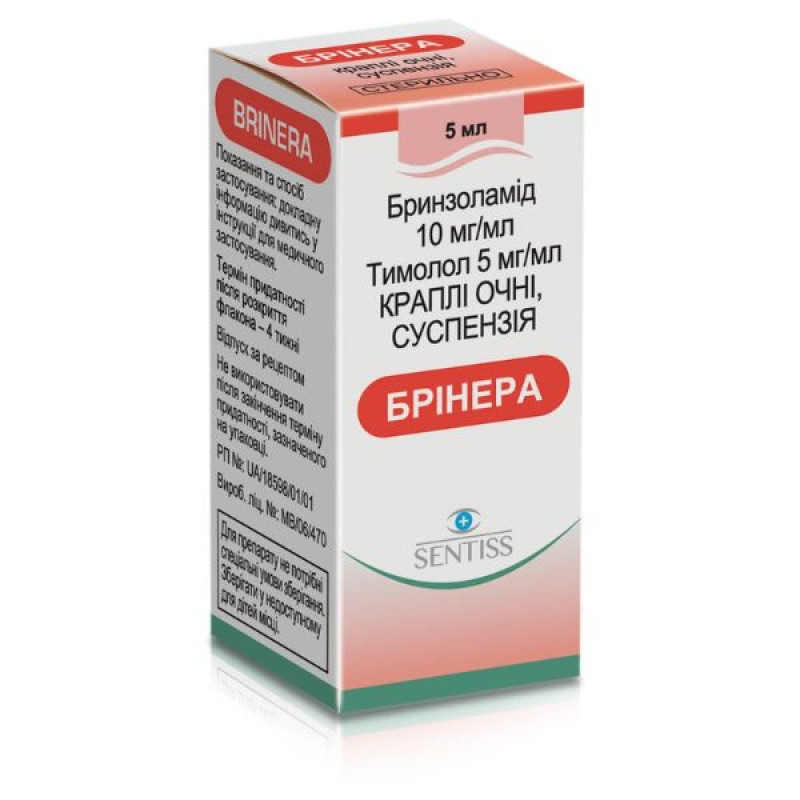
Briner is a drug used in ophthalmology. It is an antiglaucoma drug and a miotic.
Indications for use
Reduction of intraocular pressure in adult patients with open-angle glaucoma or ocular hypertension in whom monotherapy has not resulted in sufficient reduction of intraocular pressure.
Warehouse
1 ml of suspension contains brinzolamide 10 mg, timolol maleate 6.83 mg equivalent to timolol 5 mg; excipients: benzalkonium chloride, mannitol (E 421), carbomer 974P (carbomer homopolymer type B), tyloxapol, disodium edetate, sodium chloride, diluted hydrochloric acid and/or sodium hydroxide (for pH adjustment), water for injections.Contraindication
Hypersensitivity to the active substances or to any of the excipients of the drug.
Hypersensitivity to other β-blockers.
Hypersensitivity to sulfonamides.
Conditions accompanied by airway hyperresponsiveness, including bronchial asthma or a history of bronchial asthma, severe chronic obstructive pulmonary disease.
Method of application
Use in adults, including elderly patients
The dose is 1 drop of BRINERA eye drops into the conjunctival sac of the affected eye(s) 2 times daily.
Systemic absorption is reduced by applying pressure to the nasolacrimal orifice or closing the eyelids. This reduces systemic side effects and increases local activity.
If a dose is missed, treatment should be continued with the next dose according to the schedule. The dose should not exceed 1 drop in the affected eye(s) 2 times a day.
When replacing another ophthalmic antiglaucoma agent with BRINERA eye drops, the use of the other agent should be discontinued and BRINERA eye drops should be started the next day.
Application features
Brinzolamide and timolol are absorbed systemically. Due to the presence of the β-adrenergic active component timolol, the same cardiovascular, pulmonary and other adverse reactions as with systemic β-adrenergic receptor blockers may occur with the use of the drug. The incidence of systemic adverse reactions with topical ophthalmic use is lower than with systemic use.
Pregnant women
BRINERA eye drops should not be used during pregnancy.
Children
The safety and effectiveness of BRINERA eye drops in children under 18 years of age have not been established.
Drivers
BRINERA eye drops have minimal effect on the ability to drive or use machines.
Temporary blurred vision or visual disturbances may affect the ability to drive or use machines. If blurred vision occurs during instillation, the patient should wait until the vision clears before driving or using machines.
Overdose
If the contents of the vial are accidentally swallowed, symptoms of β-blocker overdose may include bradycardia, hypotension, heart failure, and bronchospasm.
Side effects
Infectious and parasitic diseases: rhinopharyngitis, pharyngitis, sinusitis, rhinitis.
From the blood and lymphatic system: decreased white blood cell count, decreased red blood cell count, increased chloride levels in the blood.
On the part of the immune system: anaphylaxis, anaphylactic shock, systemic allergic reactions, including angioedema, local and generalized rashes, hypersensitivity, urticaria, itching.
Metabolic: hypoglycemia.
Storage conditions
The drug does not require special storage conditions.
Keep out of reach of children.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.