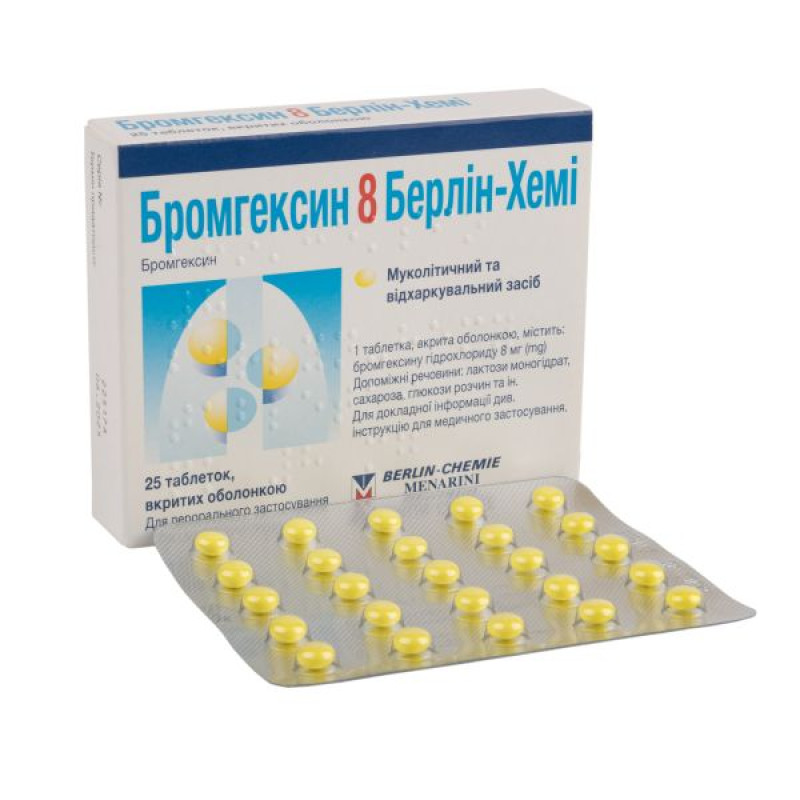
Bromhexine 8 Berlin-Chemie film-coated tablets 8 mg blister No. 25
Instructions Bromhexine 8 Berlin-Chemie film-coated tablets 8 mg blister No. 25
Composition
active ingredient: bromhexine hydrochloride;
1 film-coated tablet contains 8 mg of bromhexine hydrochloride;
excipients: lactose monohydrate, corn starch, gelatin, colloidal anhydrous silica, magnesium stearate, sucrose, calcium carbonate, light magnesium carbonate, talc, polyethylene glycol 6000, povidone K 25, glucose solution, carnauba wax, titanium dioxide (E 171), quinoline yellow dye (E 104).
Dosage form
Film-coated tablets.
Main physicochemical properties: slightly convex on both sides, film-coated tablets, from yellow to greenish-yellow in color, with an almost white core.
Pharmacotherapeutic group
Medicines used for coughs and colds. Mucolytics. ATX code R05C B02.
Pharmacological properties
Pharmacodynamics
Bromhexine is a synthetic derivative of the plant-derived active ingredient vasicin. It has secretolytic and secretomotor effects in the bronchial tract. In animal studies, it has been shown to increase the proportion of serous bronchial secretions. It is believed that mucus transport is facilitated by reducing viscosity and activating ciliated epithelium.
After the use of bromhexine, the concentrations of the antibiotics amoxicillin, erythromycin and oxytetracycline in sputum and bronchial secretions increase. The clinical significance is uncertain.
Pharmacokinetics
After oral administration, bromhexine is almost completely absorbed, with a half-life of approximately 0.4 hours. Tmax after oral administration is 1 hour. The first-pass effect is approximately 80%. Biologically active metabolites are formed. Binding to plasma proteins is 99%.
The decline in plasma concentrations is multiphasic. The half-life, which limits the duration of action, is about 1 hour. The terminal half-life is about 16 hours. This is due to the redistribution of small amounts of bromhexine from the tissues. The volume of distribution is about 7 liters per 1 kg of body weight. Bromhexine does not accumulate. Bromhexine penetrates the placenta, into the cerebrospinal fluid and into breast milk. It is excreted mainly by the kidneys in the form of metabolites formed in the liver. Due to the high protein binding and large volume of distribution, as well as the slow redistribution from the tissues into the blood, major removal of bromhexine by dialysis or forced diuresis is not expected.
In severe liver disease, the clearance of the active substance may be reduced. In severe renal failure, the possibility of an increase in the half-life of bromhexine metabolites cannot be excluded. Under physiological conditions, nitrosation of bromhexine is possible in the stomach.
Preclinical safety data
Preclinical data obtained during conventional pharmacological safety studies do not indicate the presence of a specific hazard for humans, as well as the presence of chronic toxicity, genotoxicity, carcinogenicity, toxicity to reproductive organs and development.
Chronic toxicity
Studies in various animal species (rats, mice, dogs) using very high doses and long-term treatment have not revealed any significant toxic potential for humans under normal therapeutic use.
Mutagenic and carcinogenic potential
In vitro (Ames test) and in vivo/in vitro (mammalian mediator mutagenicity test) studies did not reveal any mutagenic effects of bromhexine. Carcinogenicity studies conducted in rats did not reveal any carcinogenic potential of bromhexine.
Reproductive toxicity
Bromhexine crosses the placenta. Animal studies have not revealed any signs of teratogenic effects of bromhexine in rats, mice or rabbits. Bromhexine in therapeutic doses did not affect the development and behavior of the offspring. Bromhexine has not been shown to affect fertility.
Indication
Secretolytic therapy for acute and chronic bronchopulmonary diseases accompanied by impaired sputum formation and movement.
Contraindication
Hypersensitivity to the active substance or to any of the excipients. Bromhexine 8 Berlin-Chemie is contraindicated in patients with hereditary galactose or fructose intolerance, Lapp lactase deficiency, glucose-galactose malabsorption syndrome or hereditary sucrase-isomaltase insufficiency. Bromhexine 8 Berlin-Chemie should not be used in patients with gastric or duodenal ulcers, or with a history of peptic ulcer disease, as bromhexine may affect the gastrointestinal mucosa.
Bromhexine 8 Berlin-Chemie is contraindicated for use in children under 6 years of age due to the high content of the active substance. For this age group, medicines with a reduced dosage should be prescribed.
Interaction with other medicinal products and other types of interactions
With the combined use of Bromhexine 8 Berlin-Chemie and antitussives (cough suppressants), a dangerous increase in secretions may occur due to a violation of the cough reflex, therefore, the appropriateness of prescribing such a combination should be carefully considered and special care should be taken during therapy.
Simultaneous administration of bromhexine with antibiotics (amoxicillin, erythromycin, cefuroxime, doxycycline), sulfonamide drugs contributes to an increase in their concentration in sputum and bronchial secretion. With the simultaneous use of substances that irritate the digestive tract, a mutual increase in the irritating effect on the gastric mucosa is possible.
Application features
Skin reactions
Severe skin reactions associated with the use of bromhexine, such as erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis and acute generalized exanthematous pustulosis, have been reported. If symptoms or signs of progressive skin rash (sometimes with blistering or mucosal lesions) occur, a doctor should be consulted immediately and bromhexine should be discontinued.
Stomach or duodenal ulcer
Bromhexine 8 Berlin-Chemie should not be used in patients with gastric or duodenal ulcers or a history of peptic ulcer disease, as bromhexine may affect the mucous membrane of the gastrointestinal tract.
Lungs and respiratory tract
In case of impaired bronchial motility, accompanied by the formation of a large amount of bronchial secretion (for example, in such a rare disease as primary ciliary dyskinesia), Bromhexine 8 Berlin-Chemie should be used with extreme caution due to the possible accumulation of secretion.
Liver and kidney disorders
Bromhexine 8 Berlin-Chemie should be used with particular caution (i.e. at longer intervals or in lower doses) in patients with impaired renal function or with severe liver disease.
In severe renal failure, accumulation of bromhexine metabolites formed in the liver is possible.
Periodic monitoring of liver function is recommended, especially with prolonged use.
Lactose, glucose, sucrose
Lactose, glucose and sucrose are part of this medicinal product. Therefore, Bromhexine 8 Berlin-Chemie is contraindicated in patients with hereditary diseases such as galactose or fructose intolerance, lactase deficiency, glucose-galactose malabsorption or sucrase-isomaltase insufficiency.
Ability to influence reaction speed when driving vehicles or other mechanisms
Bromhexine 8 Berlin-Chemie has no or negligible effect on the reaction rate when driving or using other mechanisms.
Use during pregnancy or breastfeeding
Pregnancy
There is limited data on the use of bromhexine in pregnant women. Therefore, Bromhexine 8 Berlin-Chemie should only be prescribed by a doctor after a careful benefit/risk assessment and is not recommended in the first trimester of pregnancy.
Breastfeeding period
Since the active substance passes into breast milk, Bromhexine 8 Berlin-Chemie should not be used during breastfeeding.
Method of administration and doses
The film-coated tablets should be taken after meals and washed down with plenty of liquid (e.g. a glass of water).
Recommended doses of Bromhexine 8 Berlin-Chemie:
Adults and children over 14 years of age: 1–2 film-coated tablets 3 times a day (corresponding to 24–48 mg/day of bromhexine hydrochloride).
Children aged 6 to 14 years, as well as patients weighing less than 50 kg: 1 film-coated tablet 3 times a day (corresponding to 24 mg/day of bromhexine hydrochloride).
Other special patient groups: Bromhexine 8 Berlin-Chemie should be used with particular caution (i.e. at longer intervals or in lower doses) in the presence of impaired renal function or severe liver disease (see section "Special warnings and precautions for use").
The duration of treatment is determined individually, depending on the indications and the dynamics of the disease. Bromhexine 8 Berlin-Chemie should not be used for longer than 4–5 days without the appropriate recommendation of a doctor.
Children
Bromhexine 8 Berlin-Chemie is not intended for use in children under 6 years of age due to the high content of the active substance.
Overdose
Symptoms. To date, there have been no reports of life-threatening overdoses. A case of overdose of bromhexine hydrochloride in toddlers has been published, in which 4 out of 25 children experienced vomiting and 3 children experienced impaired consciousness, ataxia, diplopia, mild metabolic acidosis, and rapid breathing. No symptoms were observed in toddlers after administration of up to 40 mg of bromhexine, even without decontamination.
Therapeutic measures. In case of significant overdose, cardiovascular function should be monitored and, if necessary, symptomatic therapy should be prescribed. Due to the low toxicity of bromhexine, more invasive measures to reduce the absorption of the drug or to accelerate the elimination of bromhexine from the body are generally not indicated. In addition, given the pharmacokinetic characteristics of bromhexine (high volume of distribution, slow reverse distribution and high level of protein binding), a significant increase in the rate of drug excretion should not be expected during dialysis or forced diuresis.
Since in children aged 2 years and older, even with a significant overdose of the drug, only mild symptoms are expected to develop, measures to reduce absorption and accelerate the elimination of bromhexine may not be necessary when taking doses up to 80 mg of bromhexine hydrochloride (corresponding to 10 tablets of 8 mg); the appropriate limit in younger children is 60 mg of bromhexine hydrochloride (6 mg/kg body weight).
Caution: If the amount of bromhexine taken exceeds the above limits, possible side effects of the excipients of the medicinal product should also be taken into account (see section "Special instructions for use").
Adverse reactions
The frequency of adverse reactions is defined as follows: very common (≥ 1/10), common (≥ 1/100 to < 1/10), uncommon (≥ 1/1000 to < 1/100), rare (≥ 1/10000 to < 1/1000), very rare (< 1/10000), unknown (frequency cannot be estimated from the available data).
From the immune system.
Rare: hypersensitivity reactions.
Not known: anaphylactic reactions including anaphylactic shock, angioedema, pruritus.
From the gastrointestinal tract.
Uncommon: nausea, stomach pain, vomiting, diarrhoea.
Exacerbation of gastric or duodenal ulcer.
On the skin and subcutaneous tissue.
Rare: rash, urticaria.
Not known: severe cutaneous adverse reactions (including erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis and acute generalized exanthematous pustulosis).
General disorders.
Uncommon: fever.
Respiratory disorders, dizziness, respiratory distress, headache, increased sweating, transient increase in serum aspartate aminotransferase, chills.
If hypersensitivity reactions, anaphylactic reactions, or any skin and mucous membrane disorders occur, you should immediately discontinue use of bromhexine and consult a doctor.
Reporting of suspected adverse reactions.
Reporting of suspected adverse reactions after the registration of a medicinal product plays an important role. This allows for continued surveillance of the benefit-risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions.
Expiration date
3 years.
Do not use the medicine after the expiry date stated on the packaging.
Storage conditions
Store at a temperature not exceeding 25 ° C. Keep out of the reach of children.
Packaging
25 film-coated tablets in a blister; 1 blister in a cardboard box.
Vacation category
Without a prescription.
Producer
BERLIN-CHEMI AG.
Location of the manufacturer and its business address
Glienicker Weg 125, 12489 Berlin, Germany.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.