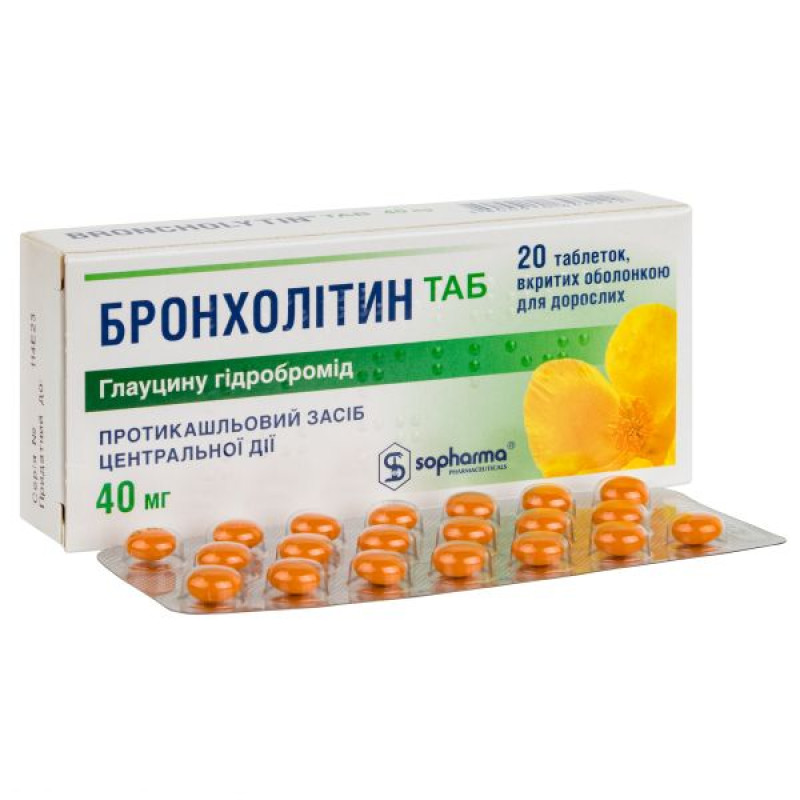
Broncholitin Tab film-coated tablets 40 mg No. 20
Instructions Broncholitin Tab film-coated tablets 40 mg No. 20
Composition
active ingredient: glaucine hydrobromide;
1 film-coated tablet contains glaucine hydrobromide 40 mg;
excipients: wheat starch, sucrose, gelatin, microcrystalline cellulose, talc, stearic acid;
shell: gelatin, titanium dioxide (E 171), sucrose, acacia, talc, macrogol 6000, glycerol, for 10 mg: Opalux pink AS-24825 (sucrose, purified water, titanium dioxide (E 171), carmine (E 120), povidone (E 1201), ponceau 4R (E 124), sodium benzoate (E 211), indigo carmine (E 132)) or for 40 mg: Opalux orange AS-23060 (sucrose, purified water, titanium dioxide (E 171), sunset yellow FCF (E 110), talc, iron oxide yellow (E 172), povidone (E 1201), sodium benzoate (E 211), indigo carmine (E 132)).
Dosage form
Film-coated tablets.
Main physicochemical properties: 40 mg tablets: biconvex, orange-colored, film-coated tablets of regular round shape with a glossy surface.
Pharmacotherapeutic group
Antitussives.
ATX code R05D B.
Pharmacological properties
Pharmacodynamics
Broncholitin Tab is a centrally acting antitussive. Contains the alkaloid glaucine, obtained from the plant Glaucinum flavum (yellow poplar), which suppresses the cough center. Unlike codeine, glaucine does not affect the respiratory center and does not cause drug dependence. It does not affect intestinal motility, exhibits weak antispasmodic activity, has a sympatholytic effect and can reduce blood pressure. It has an anti-inflammatory effect.
Pharmacokinetics
When administered orally, the drug is rapidly absorbed from the gastrointestinal tract. The time to reach maximum plasma levels is approximately 1.5 hours after administration. It is metabolized in the liver and excreted mainly in the urine.
Indication
Symptomatic treatment of dry cough of various etiologies in infectious and inflammatory diseases of the upper respiratory tract, including acute and chronic bronchitis, influenza.
Contraindication
Hypersensitivity to the active substance or to any of the excipients of the drug; arterial hypotension; acute myocardial infarction.
Interaction with other medicinal products and other types of interactions
There are no data on adverse drug interactions with Broncholitin Tab. It can be used in combination with antibiotics, chemotherapeutic agents and other drugs.
Broncholitin Tab should not be used simultaneously with cough suppressants, both central (codeine, codterpin) and peripheral (exangit, libexin) mechanisms of action. Combination with drugs that reduce bronchial secretion (e.g. atropine derivatives) is not justified.
Broncholitin Tab can be used simultaneously with bronchodilators and cardiovascular drugs.
The active ingredient is effectively combined with ephedrine, citric acid, and basil oil in combination cough relief preparations in the form of syrups.
Application features
Broncholitin Tab should not be used for productive cough accompanied by sputum production, as there is a risk of bronchial obstruction due to retention of bronchial secretions.
In case of labile blood pressure, a doctor's consultation is necessary. Broncholitin Tab should be used with caution due to the risk of collapse as a result of the sympatholytic effect of glaucine.
The drug contains, as an excipient, wheat starch, which may contain gluten, but only in small amounts, so it is considered safe for people with celiac disease.
Patients with wheat allergy (different from celiac disease) should not use this medicine.
The drug contains sucrose as an excipient in the coating. Patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase deficiency should not use Broncholitin Tab.
Dye E 110 or dye E 124 may cause allergic reactions.
Ability to influence reaction speed when driving vehicles or other mechanisms
If dizziness, headache, drowsiness, weakness, and fatigue occur during treatment with the drug, you should refrain from driving vehicles and operating machinery.
Use during pregnancy or breastfeeding
Broncholitin Tab should be used with caution in pregnant and breastfeeding women after assessing the benefit/risk ratio.
Method of administration and doses
Broncholitin Tab should be taken orally; it is recommended to use the drug after meals.
Adults
A single dose for adults is 40 mg, administered 2-3 times a day. In more severe cases, a single dose can be increased to 80 mg. The maximum daily dose should not exceed 200 mg.
Children aged 4 and over
The single dose for children aged 4 years and older is 10 mg, taken 2-3 times a day.
The maximum daily dose should not exceed 40 mg.
Patients with impaired liver and kidney function
The duration of treatment with Broncholitin Tab should not exceed 5 days.
The use of the drug for a longer period and in higher doses is possible only as prescribed and under the constant supervision of a doctor.
Children
The medicine should not be used to treat children under 4 years of age.
Overdose
Symptoms: dizziness, headache, drowsiness, weakness and fatigue, nausea, vomiting, low blood pressure.
Treatment: carry out generally accepted measures to quickly remove the drug from the body (gastric lavage, use of activated charcoal, infusion of water-salt solutions), as well as carry out symptomatic treatment.
Adverse reactions
The drug is well tolerated. Occasionally, when using a high single dose exceeding 80 mg, dizziness, headache, drowsiness, weakness and fatigue, nausea and vomiting, decreased blood pressure may occur. Allergic reactions are possible, including itching, skin rash.
If any adverse reaction becomes serious, the use of the drug should be discontinued and the doctor should be informed.
In case of adverse reactions not listed in this instruction, it is necessary to urgently inform the doctor.
Expiration date
3 years.
Storage conditions
Keep out of reach of children.
Store in the original packaging at a temperature not exceeding 25°C.
Packaging
20 film-coated tablets in a blister of PVC film and aluminum foil.
1 blister in a cardboard pack.
Vacation category
Without a prescription.
Producer
JSC "Sopharma".
JSC "VITAMINS".
Location of the manufacturer and its business address
16 Ilienskoe Shose St., Sofia, 1220, Bulgaria.
Ukraine, 20300, Cherkasy region, Uman city, Uspenska st., 31.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.