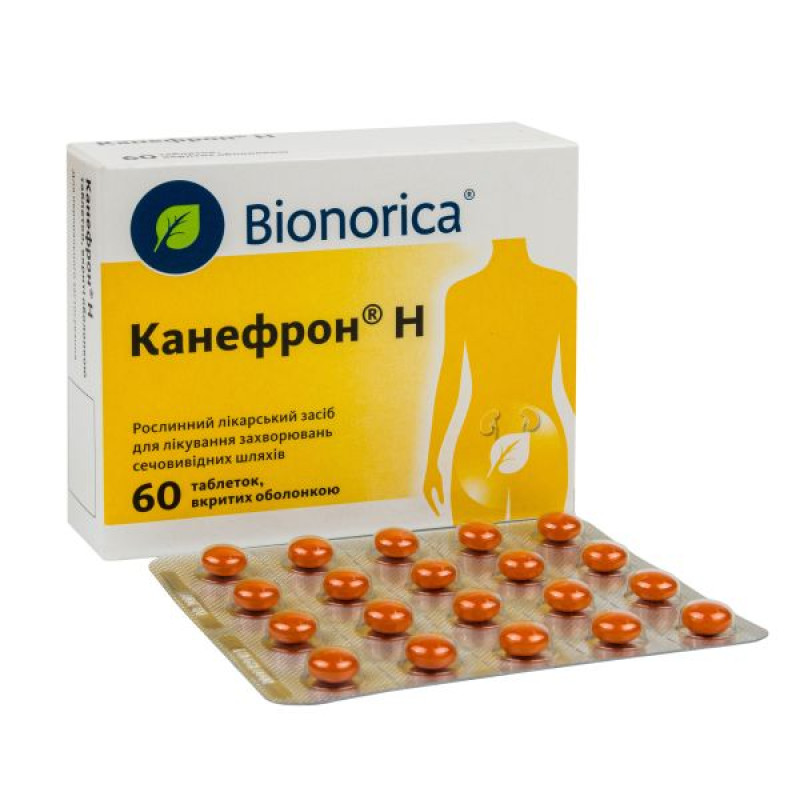
Canephron H film-coated tablets blister pack No. 60

Instructions for use Canephron N film-coated tablets blister pack No. 60
Composition
active ingredients: 1 tablet contains dried medicinal plants in powder form:
Centaury herb (Herba Centaurii) 18 mg,
lovage root (Radix Levistici) 18 mg,
rosemary leaves (Folia Rosmarini) 18 mg;
excipients: corn starch, colloidal anhydrous silica, lactose monohydrate, povidone, magnesium stearate, red iron oxide (E 172), riboflavin (E 101), calcium carbonate, dextrin, glucose syrup, glycolic montan wax, castor oil, sucrose, shellac, talc, titanium dioxide (E 171).
Dosage form
Film-coated tablets.
Main physicochemical properties: round biconvex tablets, coated with an orange shell with a smooth surface.
Pharmacotherapeutic group
Drugs used in urology. ATX code G04B X.
Pharmacological properties
Pharmacodynamics
The components that make up the herbal medicine exhibit complex activity, which is manifested in anti-inflammatory, antioxidant, antispasmodic and analgesic effects. Kanefronâ N also has antibacterial and diuretic effects, which are due to the substances contained in the herbal components of the drug.
Pharmacokinetics
Not known.
Indication
For the complex treatment of inflammatory diseases of the urinary tract.
Prevention of urinary stone formation, including after their removal.
Contraindication
Hypersensitivity to any component of the drug, or to other plants of the Apiaceae family, such as anise, fennel, and to anethole (i.e. a component of essential oils containing, for example, anise and fennel).
Peptic ulcer.
Edema due to heart failure or kidney dysfunction and/or a doctor's recommendation to limit fluid intake.
Interaction with other medicinal products and other types of interactions
Unknown.
If it is necessary to use any other medicines at the same time, you should consult a doctor.
Application features
In case of prolonged fever, cramps, blood in the urine, urination disorders, and acute urinary retention, you should immediately consult a doctor.
Patients with intolerance to some sugars should consult a doctor before taking Canephron N tablets.
The drug should not be used in patients with rare hereditary forms of fructose intolerance, galactose intolerance, lactase deficiency, glucose-galactose malabsorption syndrome or sucrase-isomaltase insufficiency.
Note for diabetics: 1 tablet contains an average of 0.02 bread units (BTU).
Ability to influence reaction speed when driving vehicles or other mechanisms
The drug does not affect the ability to drive or operate other mechanisms.
Use during pregnancy or breastfeeding
Pregnancy.
Experience in observing pregnant women (300-1000 newborns) indicates that there is no risk of fetal malformations or fetal/neonatal toxicity of Canephron® N tablets.
No signs of reproductive toxicity were observed in experimental studies.
The use of Canephron® N tablets during pregnancy is possible after consulting a doctor.
Breast-feeding.
Due to the lack of data on the excretion of Canephron H or its metabolites into breast milk, a risk to infants cannot be excluded. Therefore, the drug should not be used during breastfeeding.
Method of administration and doses
Unless otherwise prescribed by a doctor, the drug should be taken by adults and children over 12 years of age, 2 tablets 3 times a day (total daily dose: 6 tablets).
The tablets should be swallowed without chewing, with sufficient liquid (e.g. a glass of water).
While taking the drug, it is necessary to ensure the consumption of sufficient amounts of fluid.
The duration of treatment is determined by the doctor individually. If the drug is well tolerated, it can be prescribed for a long period.
Children
The drug is not recommended for use in children under 12 years of age.
Overdose
Cases of drug poisoning due to overdose are unknown.
Therapy: symptomatic.
Adverse reactions
Gastrointestinal disturbances (nausea, vomiting, diarrhea) are common. Allergic reactions may occur in case of hypersensitivity to the components of the drug, including urticaria, itching, and skin hyperemia.
If any adverse reactions occur, discontinue use of the drug and consult a doctor.
Expiration date
4 years.
Do not use the medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month.
Storage conditions
Store in original packaging out of the reach of children.
This medicinal product does not require any special temperature storage conditions.
Packaging
20 tablets in a blister; 3 blisters in a cardboard box.
Vacation category
Without a prescription.
Producer
Bionorica CE
Location of the manufacturer and its business address
Kerchensteinerstrasse, 11-15, 92318, Neumarkt, Germany
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.