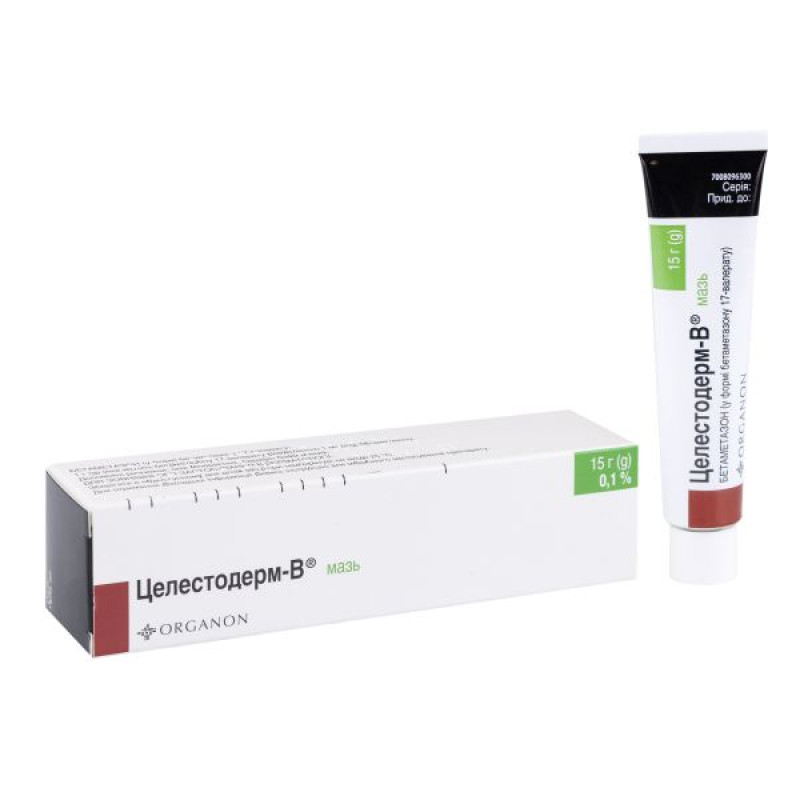
Celestoderm-B ointment 0.1% tube 15 g
Instructions Celestoderm-B ointment 0.1% tube 15 g
Composition
active ingredient: betamethasone;
1 g of ointment contains betamethasone 17-valerate equivalent to 1 mg of betamethasone;
excipients: white soft paraffin, mineral oil.
Dosage form
Ointment.
Main physicochemical properties: smooth, homogeneous white ointment without foreign inclusions.
Pharmacotherapeutic group
Corticosteroids for use in dermatology. Active corticosteroids (group III). Betamethasone. ATX code D07A C01.
Pharmacological properties
Pharmacodynamics
The pharmacodynamic activity of the drug Celestoderm-B is directly proportional to the activity of betamethasone valerate and its derivatives.
Betamethasone 17-α valerate is an ester of betamethasone. Betamethasone is a derivative of cortisol that has 1,2 double bonds, fluorine, and beta-methyl radicals. This results in higher glucocorticosteroid/anti-inflammatory but lower mineralocorticoid activity compared to hydrocortisone.
Celestoderm-B suppresses inflammation, itching, and causes vasoconstriction. The action of this drug is expected to occur after binding to steroid receptors.
Pharmacokinetics
Topical corticosteroids can be absorbed through intact skin. Absorption is increased by inflammation and/or other conditions. In particular, absorption of topical corticosteroids is increased by the use of an occlusive dressing. Therefore, the use of an occlusive dressing in resistant dermatoses increases the therapeutic effect.
Absorption and distribution
The pharmacokinetics of topical corticosteroids absorbed through the skin are the same as those of systemic administration.
Corticosteroids bind to plasma proteins to varying degrees.
Biotransformation and excretion
Primary metabolism of corticosteroids occurs in the liver, followed by renal excretion. Some topical corticosteroids and their metabolites are excreted in the bile.
Indication
Topical treatment of dermatitis, especially allergic dermatitis, toxic eczema, atopic dermatitis and psoriasis.
Contraindication
hypersensitivity to the active substances or to any of the excipients listed in the "Composition" section; rosacea; acne vulgaris; generalized plaque psoriasis; perioral dermatitis; viral skin infections such as herpes simplex or chickenpox; skin lesions caused by fungal or bacterial infection, in the absence of appropriate therapy for the infection.
It is not recommended to use Celestoderm-B ointment under occlusive dressings (plaster, etc.).
Celestoderm-B ointment should not be used in the first trimester of pregnancy (see section “Use during pregnancy or breastfeeding”).
Interaction with other medicinal products and other types of interactions
There is no information regarding the interaction of the drug Celestoderm-B, ointment, with other drugs.
Application features
If irritation or signs of hypersensitivity occur during use of Celestoderm-B, treatment with the drug should be discontinued and appropriate therapy should be prescribed.
If concomitant antifungal/antibacterial therapy is prescribed for the presence of infection and a favorable response does not occur, corticosteroids should be discontinued until signs of infection resolve.
Adverse reactions, including adrenal suppression, may occur with systemic corticosteroids. Adverse reactions may also occur with topical corticosteroids, especially in children and infants.
Systemic absorption of topical corticosteroids increases with increasing body surface area treated. Therefore, certain precautions should be taken, especially with long-term treatment and when used in children and infants.
Due to the risk of simple glaucoma and subcapsular cataracts, Celestoderm-B is not intended for use in the eye area or on the eyelids.
Prolonged use of potent topical corticosteroids may cause atrophic changes, particularly on the face; other areas of the body are less sensitive. This should be borne in mind in long-term treatment of conditions such as psoriasis, atopic dermatitis and severe eczema. Facial use should never exceed 5 days; occlusive dressings should not be used.
Vision impairment
Visual disturbances are possible with systemic and topical corticosteroids, including intranasal, inhaled, and ophthalmic administration. If symptoms such as blurred vision or other visual disturbances occur, the patient should be evaluated by an ophthalmologist to evaluate possible causes of visual disturbances, which may include cataracts, glaucoma, or rare conditions such as central serous chorioretinopathy, which have been reported following the use of systemic and topical corticosteroids.
Children.
Children receiving corticosteroids have been reported to have hypothalamic-pituitary-adrenal (HPA) axis suppression, Cushing's syndrome, growth retardation, failure to gain weight, and increased intra-abdominal pressure. Adrenal cortex suppression in children includes low plasma cortisol and failure to respond to adrenocorticotropic hormone (ACTH) stimulation test. Fontanelle protrusion, headache, and bilateral optic disc edema are manifestations of increased intracranial pressure.
Ability to influence reaction speed when driving vehicles or other mechanisms
The drug Celestoderm-B does not affect the patient's reaction speed when driving or working with other mechanisms, or this effect is insignificant.
Use during pregnancy or breastfeeding
Pregnancy
The use of topical corticosteroids in pregnant women has not been studied. Drugs in this group should be used during pregnancy only if the expected benefit outweighs the potential risk to the fetus. Drugs in this group should not be used in pregnant women in large amounts or for long periods. Celestoderm-B ointment should not be used in the first trimester of pregnancy.
Breast-feeding
It is not known whether corticosteroids are absorbed after topical application. Levels in breast milk are also unknown. Therefore, either breast-feeding or the drug should be discontinued, taking into account the importance of the drug to the mother.
Method of administration and doses
Doses
Adult patients, including elderly patients
Apply 1-3 times a day. The frequency of application is determined depending on the severity of the disease. For mild diseases, application once a day is sufficient, while for severe forms of the disease, application 3 times a day is necessary.
Patients with renal or hepatic insufficiency
There are no data on the use of Celestoderm-B in patients with renal or hepatic insufficiency.
Method of application
For cutaneous use. Apply a thin layer to affected areas of skin.
Children
Celestoderm-B is not recommended for use in children and adolescents due to the lack of clinical data on efficacy and safety for use in this age group.
Overdose
Symptoms
Excessive and prolonged use of topical corticosteroids may cause suppression of pituitary-adrenal function, leading to secondary adrenal insufficiency, hypercorticism, and Cushing's syndrome.
Treatment
In case of overdose, appropriate symptomatic treatment is indicated. Symptoms of acute hypercorticism are usually reversible. If necessary, correction of electrolyte balance is carried out. In case of signs of chronic toxicity, gradual withdrawal of the corticosteroid is recommended.
Adverse reactions
Adverse reactions are listed by system organ class and frequency. The frequency of adverse reactions is defined according to the following values: very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥ 1/1,000 to < 1/100); rare (≥ 1/10,000 to < 1/1,000); very rare (< 1/10,000); not known (frequency cannot be estimated from the available data).
Local adverse reactions associated with the use of topical corticosteroids, and especially when used under occlusive dressings, may include:
Adverse reactions
Blurred vision (see also section "Special warnings and precautions for use")
| Frequency | |
| Infections and infestations | |
| Unknown | Folliculitis, secondary infection |
| Visual impairment | |
| Unknown | |
| General disorders and administration site conditions | |
| Unknown | Dry skin at the application site, irritation at the application site |
| Skin and subcutaneous tissue disorders | |
| Unknown | Pruritus, hypertrichosis, dermatitis acneiform, skin hypopigmentation, contact dermatitis, perioral dermatitis, skin maceration, skin atrophy, skin striae, sweating, skin burning sensation |
Reporting of suspected adverse reactions
It is important to report suspected adverse reactions after a medicinal product has been authorised. This allows for continued monitoring of the benefit/risk balance of the medicinal product. Qualified healthcare professionals are asked to report all suspected adverse reactions.
Expiration date
3 years.
Storage conditions
Store out of the reach of children at a temperature not exceeding 25 °C.
Packaging
15 g in an aluminum tube. 1 tube in a cardboard box.
Vacation category
According to the recipe.
Producer
Schering-Plough Labo N.V.
Location of the manufacturer and its business address
Industrial Park 30, Heist-op-den-Berg, 2220, Belgium.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.