Cellophane capsules 10 mg blister No. 20
Instructions for use Cellophane capsules 10 mg blister No. 20
Composition
active ingredient: zaleplon;
1 capsule contains zaleplon 10 mg;
excipients: lactose monohydrate, microcrystalline cellulose, colloidal anhydrous silica, sodium lauryl sulfate, sodium starch glycolate (type A), magnesium stearate; capsule composition: gelatin, titanium dioxide (E 171), red iron oxide (E 172), black iron oxide (E 172), erythrosine (E 127), indigo carmine (E 132).
Dosage form
Capsules.
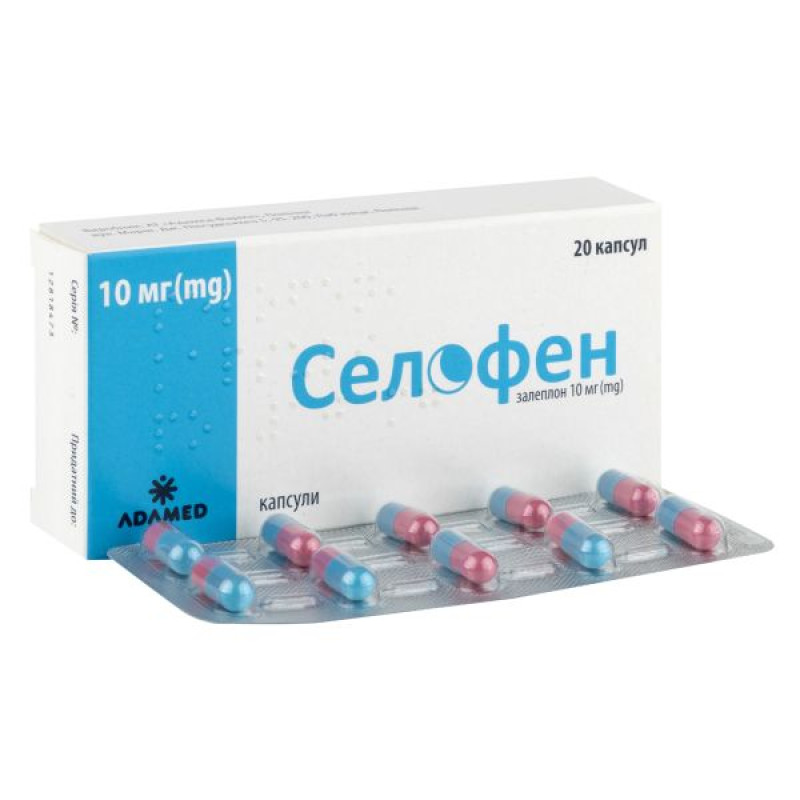
Main physicochemical properties: hard gelatin capsules with a pink body and a blue cap, size "3", containing white powder.
Pharmacotherapeutic group
Sleeping pills and sedatives. ATX code N05C F03.
Pharmacological properties
Pharmacodynamics
Zaleplon is a drug from the pyrazolopyrimidine group, which is different from benzodiazepines and other hypnotics. Zaleplon interacts with the benzodiazepine GABA receptor, which is found in neuronal structures of the central nervous system (CNS).
The pharmacokinetic profile of zaleplon demonstrates rapid absorption and elimination. It selectively binds to type I benzodiazepine receptors.
Pharmacokinetics
Absorption
Zaleplon is rapidly and almost completely absorbed from the gastrointestinal tract (at least 71%) after oral administration. Maximum serum concentrations (Cmax) are reached within approximately 1 hour. Zaleplon is biotransformed in the liver, and its bioavailability is approximately 30%.
Distribution
Zaleplon is lipophilic and its volume of distribution after intravenous administration is approximately 1.4 ± 0.3 L/kg. In vitro, it is approximately 60% bound to plasma proteins, indicating a low risk of drug interactions due to protein binding.
Biotransformation
Zaleplon is biotransformed mainly in the liver to 5-hydroxy-zaleplon under the action of the enzyme aldehyde oxidase. To a lesser extent, it is transformed by the CYP3A4 isoenzyme to diethylzaleplon and then to 5-hydroxy-diethylzaleplon. Then both metabolites, 5-hydroxy-zaleplon and 5-hydroxy-diethylzaleplon, are transformed to glucuronides and excreted in the urine. In vitro studies in animals have shown that the metabolites of zaleplon are pharmacologically inactive. The concentration of zaleplon in the blood plasma increases linearly, no accumulation of zaleplon was detected after administration of up to 30 mg/day. The half-life of zaleplon is approximately 1 hour.
Breeding
Zaleplon is excreted as inactive metabolites, mainly in the urine (71%) and feces (17%). The majority (57%) of the administered dose is excreted in the urine as 5-hydroxy-zaleplon and its glucuronide derivatives, with another 9% excreted as 5-hydroxy-diethylzaleplon and its glucuronide derivatives. The remainder of the dose is made up of minor metabolites excreted in the urine. The majority of metabolites recovered in the feces are 5-hydroxy-zaleplon.
Indication
A severe form of sleep disorder, manifested by difficulty falling asleep.
Contraindication
Hypersensitivity to the components of the drug. Breastfeeding period. Severe liver failure. Severe respiratory failure. Severe renal dysfunction. Night apnea syndrome. Myasthenia gravis. Children's age.
Interaction with other medicinal products and other types of interactions
Alcohol - simultaneous use with zaleplon is not recommended due to increased sedative effects. This limits mental and physical reactions and reduces the ability to drive and operate mechanical devices.
Caution should be exercised when used simultaneously with drugs that affect the central nervous system. The central sedative effect may be enhanced when combined with the following drugs: drugs used in mental illnesses (antipsychotics, hypnotics, anxiolytics, sedatives, antidepressants), drugs used in the treatment of severe pain (opioid analgesics), drugs for the treatment of seizures (antiepileptic drugs), anesthetic drugs, drugs used in the treatment of allergies (antihistamines with sedative effects).
Venlafaxine (75 mg or 150 mg/day, extended-release formulation) coadministered with 10 mg zaleplon did not impair memory (immediate and delayed word recall) or psychomotor performance (digit substitution test). Furthermore, no pharmacokinetic interaction was observed between zaleplon and venlafaxine (extended-release formulation).
When used with opioid analgesics, the euphoric effect may be enhanced, leading to increased physical dependence.
Cimetidine (a non-specific, moderately potent inhibitor of hepatic enzymes such as oxidase and CYP3A4) increases zaleplon plasma concentrations by 85% by inhibiting aldehyde oxidase and CYP3A4 (enzymes responsible for zaleplon metabolism). Therefore, caution should be exercised when these drugs are used concomitantly.
The use of the drug Cellofen simultaneously with 800 mg of erythromycin (a strong selective CYP3A4 inhibitor) leads to an increase in the concentration of zaleplon in the blood plasma by
Rifampicin, as a strong inducer of liver enzymes, such as CYP3A4, can cause a four-fold decrease in zaleplon concentrations. CYP3A4 inducers, such as rifampicin, carbamazepine, phenobarbital, when used simultaneously with Selofen, can reduce the effectiveness of zaleplon.
Zaleplon does not affect the pharmacokinetics and pharmacodynamics of digoxin and warfarin with a narrow therapeutic index.
Ibuprofen, when used simultaneously with zaleplon, does not cause additional interactions.
Application features
The course of treatment should be as short as possible, the maximum duration can be 2 weeks.
The use of Cellophane may lead to mental and physical dependence. The risk of developing dependence increases in proportion to the dose and duration of treatment. It is also higher in patients who abuse alcohol and sleeping pills.
The patient should be informed about the possibility of insomnia recurring after the end of treatment.
In patients taking hypnotics and sedatives, behavioral disorders in a state of partial consciousness, with reversible amnesia, have been observed after their use. These phenomena can occur in patients who have not been previously treated or who have been treated with hypnotics and sedatives. A phenomenon such as driving while half asleep can be observed when using only hypnotics and sedatives in therapeutic doses, but the risk of such a condition increases with the use of alcohol and other substances that have a depressant effect on the central nervous system, and in the case of taking doses that exceed the maximum recommended dose. Due to the threat to the patient and others, it is recommended to refuse the use of zaleplon in patients who have previously experienced such a phenomenon as driving while half asleep. Other behavioral disorders (e.g., preparing and eating food, talking on the phone, sexual intercourse while half asleep) have also been observed after taking hypnotics and sedatives. Patients usually do not remember these events.
Severe anaphylactic and anaphylactoid reactions have been reported with hypnotics and sedatives, including zaleplon. Angioedema involving the tongue, glottis, and larynx has been reported after the first dose or subsequent doses. Some patients taking hypnotics and sedatives have experienced additional symptoms such as dyspnea, throat spasm, or nausea and vomiting. Some patients have required hospitalization and emergency treatment. If angioedema involves the tongue, glottis, and larynx, upper airway obstruction may occur, which can be fatal. Zaleplon should not be re-administered to patients who have experienced angioedema following its use.
Insomnia may be caused by physical or mental disorders. Insomnia that does not resolve or worsens after short-term use of zaleplon may indicate the need for re-evaluation of the patient.
The half-life of zaleplon is short, about 1 hour. If the patient wakes up early in the morning, alternative therapy should be considered. The patient should be advised not to take a second dose of Zelofene that same night.
The use of zaleplon with other drugs that affect CYP3A4 may alter zaleplon concentrations.
The drug contains lactose monohydrate. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or lactose-galactose malabsorption should not take Cellofen.
Habituation: Taking benzodiazepines and short-acting benzodiazepine-like drugs for several weeks may be accompanied by a decrease in the hypnotic effect.
Dependence. The development of physical and mental dependence is possible, the likelihood of which is associated with taking large doses of the drug, long-term treatment, and the presence of alcohol and drug dependence.
In case of physical dependence, abrupt withdrawal of the drug leads to the development of withdrawal symptoms: headache and muscle pain, severe anxiety, increased tension, restlessness, confusion and irritability. In severe cases, derealization, depersonalization, hyperacusis, paresthesia of the extremities, increased reaction to light, sound and physical stimuli, hallucinations, epileptic seizures are possible. In post-marketing studies, reports of the development of dependence associated with the use of zaleplon were received, mainly in combination with other psychotropic substances.
Memory and psychomotor functions. Anterograde amnesia and psychomotor dysfunction may develop, which most often occur several hours after taking the drug. In order to prevent the development of these symptoms, the drug should be taken only if the patient has the opportunity to sleep continuously, at least for the first 4 hours after taking the drug.
Psychiatric and paradoxical reactions: Zaleplon treatment should be discontinued in the event of increased anxiety, agitation, irritability, aggression, loss of control, perceptual disturbances, delusions, fits of rage, nightmares, depersonalization, hallucinations, psychosis, extraversion and especially out-of-character behavioral disturbances. Children and elderly patients are most susceptible to developing such symptoms.
Use of the drug Cellophane in the elderly.
Zaleplon can be used in the elderly. The pharmacokinetics of zaleplon in them do not differ significantly from those in younger people. A dosage of 5 mg is recommended due to the greater sensitivity to the effects of hypnotics.
Use of the drug Cellophane in patients with impaired liver function
Patients with severe hepatic impairment should not be given zaleplon. Patients with mild to moderate hepatic impairment require dose adjustment due to increased bioavailability of zaleplon.
Use of the drug Cellophane in patients with impaired renal function.
In subjects with renal insufficiency, the pharmacokinetics of zaleplon do not differ significantly from those in healthy subjects, but they are more likely to have increased concentrations of inactive metabolites of zaleplon.
Respiratory failure.
Particular caution should be exercised when prescribing Cellophane to patients with chronic respiratory failure.
Psychoses
The drug and benzodiazepine derivatives should not be used for the basic treatment of psychosis.
Use in individuals who abuse alcohol or drugs
Great caution should be exercised when using benzodiazepines and drugs with similar effects in this group of patients. Alcohol should not be consumed during treatment.
Depression
Benzodiazepines and drugs with similar effects should not be used as monotherapy for depression or anxiety associated with depression. Due to the greater risk of deliberate overdose in depressed individuals, the amount of drug prescribed to these patients, including zaleplon, should be limited to the minimum necessary.
Use during pregnancy or breastfeeding
Pregnancy
Pregnant women should not take zaleplon due to the lack of clinical studies involving pregnant women.
Women of childbearing potential should be advised to discontinue treatment with Cellofen if a planned or suspected pregnancy occurs.
If, for medical reasons, Selofen is prescribed by a doctor during the last months of pregnancy or during childbirth, the effect of zaleplon on the newborn should be expected. In this case, hypothermia, arterial hypotension or moderate respiratory depression may occur. There is a risk that infants of women who took the drug for a long time during pregnancy (in the last few weeks of pregnancy) will have physical dependence and the risk of developing a withdrawal syndrome.
Lactation
Due to the penetration of the drug into breast milk, the use of zaleplon in breastfeeding women is contraindicated.
The ability to influence the reaction speed when driving vehicles or other mechanisms.
The drug significantly affects the ability to drive and use machines. Drowsiness, amnesia, impaired concentration, and impaired muscle function impair the ability to drive and use machines. In case of insufficient sleep, the likelihood of impaired attention increases.
Patients whose activities require significant psychophysical activity should also be careful.
Cellophane affects the speed of psychomotor reactions, so it should not be prescribed to patients whose activities require increased attention and significant psychomotor activity.
Method of administration and doses
Cellophane is intended for use by adults.
The recommended daily dose is 10 mg. A second dose should not be taken on the same night. The maximum daily dose is 10 mg. The maximum duration of treatment is 2 weeks.
For individuals with mild or moderate hepatic insufficiency, renal insufficiency, or chronic respiratory failure, a daily dose of 5 mg is recommended.
Zaleplon should not be taken with or after food, as this delays the absorption of the drug. You should not drink alcohol while taking zaleplon.
Cellophane should be applied immediately before going to bed and at least 4 hours before waking up.
Elderly patients
A daily dose of 5 mg is recommended, given the high sensitivity to hypnotics. If this dosage cannot be achieved, zaleplon should not be used in such patients.
In case of liver dysfunction
Due to reduced clearance, patients with mild to moderate hepatic impairment should be given a 5 mg dose. If this dosage cannot be achieved, zaleplon should not be used in such patients. The drug is contraindicated in patients with severe hepatic impairment.
In case of kidney dysfunction
The pharmacokinetics of the drug do not change in patients with mild or moderate renal impairment, therefore there is no need for dose adjustment of the drug.
Currently, there is insufficient clinical data regarding the use of zaleplon in children, so the drug is contraindicated in this category of patients.
Overdose
There is currently insufficient data on zaleplon overdose. Symptoms of overdose are associated with an increase in the drug's effects, leading to CNS depression ranging from drowsiness to coma.
Symptoms of mild overdose: drowsiness, disorientation, lethargy.
In case of severe overdose
may occur: ataxia, decreased general muscle tone, hypotension, respiratory depression, rarely coma, very rarely - fatal outcome. Overdose of zaleplon is life-threatening for people taking other CNS depressants (including alcohol).
In case of oral overdose of benzodiazepines and drugs with similar action, vomiting should be induced (within one hour) if the patient is conscious, or gastric lavage should be performed, ensuring a patent airway if the patient is unconscious. If gastric lavage is ineffective, activated charcoal should be used to minimize absorption of the drug. Symptoms of overdose are associated with an increase in the effect of the drug, which leads to inhibition of the central nervous system, from drowsiness to coma.
Treatment: supportive and symptomatic therapy is indicated.
If the patient is conscious, induce vomiting or perform gastric lavage and
administer a sorbent to minimize absorption of zaleplon. Flumazenil has been shown to be an effective antidote in animal studies, but there is no data on its effectiveness in humans.
Chromaturia has been observed following zaleplon overdose.
Adverse reactions
The following are the side effects and their frequency of occurrence that were observed during clinical trials of zaleplon.
Systemic disorders
Uncommon (>1/1000, <1/100): anorexia, asthenia, decreased tactile sensitivity, malaise, photosensitivity.
Central nervous system (CNS)
Common (>1/100, <1/10): amnesia, paresthesia, drowsiness, disorientation.
Uncommon (>1/1000, <1/100): ataxia/discoordination, apathy, depersonalization, depression, dizziness, hallucinations, hearing impairment, olfactory hallucinations, diplopia, incoherent speech, visual field defects, depression, psychotic and paradoxical reactions, decreased concentration, hypoesthesia, amnesia.
Frequency not determined: somnambulism.
From the organs of vision
Uncommon (>1/1000, <1/100): visual disturbances, diplopia.
From the hearing organs
Uncommon (>1/1000, <1/100): hearing impairment.
Gastrointestinal tract
Uncommon (>1/1000, <1/100): nausea.
Frequency not determined: increased liver transaminases.
From the reproductive system
Often (>1/100, <1/10): algodismenorrhoea.
On the part of the immune system
Very rare (<1/10,000): anaphylactic and pseudoanaphylactic reactions.
Frequency not determined: angioedema.
Skin and subcutaneous tissue disorders
Uncommon (>1/1000, <1/100): photosensitivity reactions.
Frequency unknown: angioedema.
Metabolism and nutrition
Uncommon: anorexia.
General disorders and administration site conditions
Uncommon: asthenia, malaise.
Liver and biliary tract
Frequency unknown: hepatotoxicity (as increased aminotransferase activity).
Mental disorders
Uncommon: depersonalization, hallucinations, depression, confusion, apathy, confusional state.
Frequency unknown: somnambulism.
Amnesia
Amnesia may occur even at therapeutic doses. The risk of amnesia increases with high doses. Amnesia may be associated with atypical behavior.
Depression
During the use of the drug Selofen, a relapse of pre-existing depression may occur.
Expiration date
4 years.
Storage conditions
Store at a temperature not exceeding 25 °C in the original packaging. Keep out of the reach of children.
Packaging
10 capsules in a blister; 1 or 2 blisters in a cardboard box.
Vacation category
According to the recipe.
Producer
Pabianice Pharmaceutical Plant Polfa A.T., Poland.
Location of the manufacturer and its business address
St. Marsh. J. Pilsudskiego 5, 95-200, Pabianice, Poland.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.