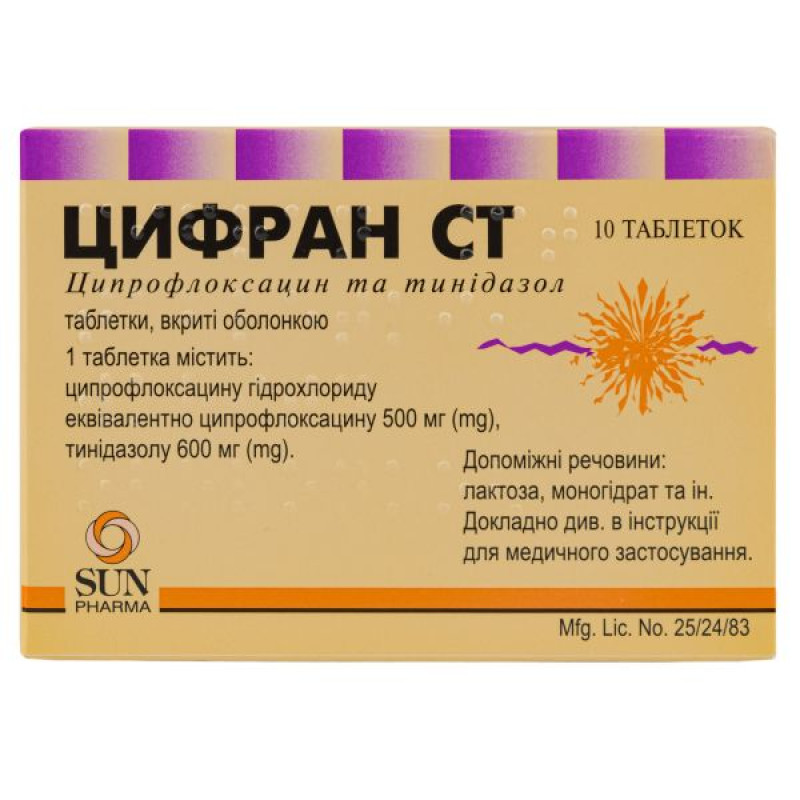
Cifran ST film-coated tablets blister pack No. 10
Instructions for use Cifran ST film-coated tablets, blister pack No. 10
Composition
active ingredients: ciprofloxacin, tinidazole;
1 film-coated tablet contains ciprofloxacin hydrochloride equivalent to ciprofloxacin 500 mg; tinidazole 600 mg;
excipients: microcrystalline cellulose, colloidal anhydrous silicon dioxide, magnesium stearate, sodium starch glycolate (type A), sodium lauryl sulfate, talc;
shell: Opadry yellow 31F 52949 [hypromellose; lactose monohydrate; titanium dioxide (E 171); macrogol 4000; iron oxide yellow (E 172)].
Dosage form
Film-coated tablets.
Main physicochemical properties: yellow, oblong, film-coated tablets with a break line on one side.
Pharmacotherapeutic group
Antimicrobial agents for systemic use.
Combined antibacterial agents. Fluoroquinolones in combination with other antibacterial agents. ATX code J01R A04.
Pharmacological properties
Pharmacodynamics
Ciprofloxacin inhibits the enzyme DNA gyrase, which plays an important role in the process of segmental despiralization and spiralization of the chromosome during the bacterial reproduction phase and prevents chromosomal transcription of information necessary for normal metabolism of the bacterial cell, which leads to inhibition of the pathogen's ability to reproduce. The drug has a rapid and pronounced bactericidal effect on microorganisms in both the reproduction phase and the resting phase. It is highly effective against almost all gram-negative and gram-positive pathogens. Escherichia coli, Shigella spp., Salmonella spp., Citrobacter spp., Klebsiella spp., Enterobacter spp., Serratia spp., Hafnia spp., Edwardsiella spp., Proteus (both indole-positive and indole-negative strains), Morganella spp., Providencia spp., Yersinia, Vibrio spp., Aeromonas spp., Plesiomonas, Pasteurela, Haemophillus, Campylobacter spp., Pseudomonas spp. (including Pseudomonas aeruginosa), Legionella, Moraxella spp., Branhamella spp., Acinetobacter spp., Brucella spp., Staphylococcus spp., Listeria spp., Corinebacterium, Chlamidia, as well as plasmid forms of bacteria are sensitive to ciprofloxacin.
As shown in in vitro studies, as well as when using a surrogate marker, ciprofloxacin is active against Bacillus anthracis.
Neisseria spp., Gardnerella spp., Flavobacterium spp., Alcaligenes spp., Streptococcus agalactiae, Enterococcus faecalis, Streptococcus pyogenes, Streptococcus pneumoniae, Streptococcus viridans, Mycoplasma hominis, Mycobacterium tuberculosis, Mycobacterium fortuitum show different sensitivity.
Anaerobic cocci (Peptococcus, Peptostreptococcus) are moderately sensitive to ciprofloxacin, and Bacteroides are resistant. Cifran ST is effective against bacteria that produce beta-lactamase. The drug is also active against microorganisms resistant to almost all antibiotics, sulfonamides and nitrofuran drugs. In some cases, Cifran ST is active against strains of microorganisms resistant to other drugs of the fluoroquinolone group. However, it should be borne in mind that there is cross-resistance between different fluoroquinolones. As a rule, Enterococcus faecium, Ureaplasma urealiticum, Nocardia asteroides, Treponema pallidum are resistant to the drug. Resistance to ciprofloxacin develops slowly and gradually ("multi-stage" type).
The prevalence of resistant strains may vary geographically and may also change over time. Local information on the susceptibility of microorganisms to ciprofloxacin should be used, especially in cases of treatment of severe infections. The information provided only provides an approximate indication of the susceptibility and resistance of certain microorganisms to ciprofloxacin.
Tinidazole is a 5-nitroimidazole derivative with a substituted imidazole moiety, active against anaerobic bacteria and protozoa. The mechanism of action of tinidazole on anaerobic bacteria and protozoa is associated with the penetration of the drug into the cells of microorganisms and with damage to DNA or inhibition of its synthesis.
Tinidazole is active against both protozoa and obligate anaerobic bacteria.
Protozoa susceptible to tinidazole include Trichomonas vaginalis, Entamoeba histolytica, and Giardia lamblia.
Tinidazole is active against Gardnerella vaginalis and most anaerobic bacteria, including Bacteroides fragilis, Bacteroides melaninogenicus, Bacteroides spp., Clostridium spp., Eubacterium spp., Fusobacterium spp., Peptococcus spp., Peptostreptococcus spp., and Veillonella spp.
Pharmacokinetics
Maximum plasma concentrations are reached after 60–20 minutes. The bioavailability of the drug is about 70–80%. The volume of distribution at steady state is 2–3 l/kg. Since the binding of ciprofloxacin to proteins is insignificant (20–30%), and the substance is found in the blood plasma mainly in a non-ionized form, almost the entire amount of the administered drug can freely diffuse into the extravasal space. In this regard, the concentration of ciprofloxacin in some body fluids and tissues can many times exceed the level of the drug in the blood serum (in particular, a high concentration is noted in bile). Ciprofloxacin is excreted mainly by the kidneys (about 45% - in unchanged form, about 11% - in the form of metabolites). The rest of the dose is excreted through the intestines (about 20% - in unchanged form, about 5–6% - in the form of metabolites). Renal clearance is 3–5 ml/min/kg, total clearance is 8–10 ml/min/kg. The half-life is 3–5 hours. Due to the fact that the drug is excreted by different routes, an increase in the half-life is observed only with significant renal dysfunction (this indicator may increase to 12 hours).
Tinidazole is rapidly and completely absorbed when administered orally.
In studies of healthy volunteers given 2 g of tinidazole orally, serum concentrations peaked at 40–51 μg/mL within 2 hours and declined to 11–19 μg/mL after 24 hours.
Plasma levels declined slowly; tinidazole was detected in plasma (at concentrations up to 1 μg/mL) 72 hours after oral administration. The plasma half-life of tinidazole is 12–14 hours.
Tinidazole is actively distributed to all tissues of the body and penetrates the blood-brain barrier. It is found in all tissues in therapeutically effective concentrations. The apparent volume of distribution is approximately 50 liters. Almost 12% of tinidazole in blood plasma is bound to proteins.
Tinidazole is excreted by the liver and kidneys. Studies in healthy volunteers have shown that within 5 days 60-65% of the administered dose is excreted by the kidneys, with 20-25% excreted unchanged. Approximately 5% of the dose is excreted in the feces.
Studies in patients with renal insufficiency (creatinine clearance below 22 ml/min) indicate that the pharmacokinetics of tinidazole in such patients do not change significantly.
The combination of ciprofloxacin with tinidazole does not affect the pharmacokinetics of these active substances.
The combination of ciprofloxacin and tinidazole enhances the antibacterial effect of the drug and significantly expands the spectrum of action on microorganisms. Cifran ST is effective against aerobic-anaerobic infections, as well as mixed protozoal-bacterial infections.
Indication
Treatment of mixed infections caused by sensitive anaerobic and aerobic microorganisms: chronic sinusitis, lung abscess, empyema, intra-abdominal infections, inflammatory gynecological diseases, postoperative infections with the possible presence of aerobic and anaerobic bacteria, chronic osteomyelitis, skin and soft tissue infections, "diabetic foot" ulcers, pressure sores, oral cavity infections (including periodontitis and periostitis).
Treatment of diarrhea or dysentery of amoebic or mixed (amebic and bacterial) etiology.
Contraindication
Hypersensitivity to ciprofloxacin or other fluoroquinolones, hypersensitivity to tinidazole or other 5-nitroimidazole derivatives, as well as to any component of the drug.
Concomitant use with tizanidine.
Organic neurological disorders, blood diseases (or a history of such), pregnancy or breastfeeding, childhood.
Interaction with other medicinal products and other types of interactions
Caution should be exercised when using Cifran ST simultaneously with class Ia or III antiarrhythmic drugs, macrolides, tricyclic antidepressants and antipsychotics, since ciprofloxacin may increase QT interval prolongation.
The simultaneous use of ciprofloxacin preparations with iron preparations, phosphate-binding polymers (e.g. sevelamer), sucralfate and antacids containing magnesium, aluminum, calcium, and drugs with a high buffer capacity (e.g. antiretrovirals) reduces the absorption of ciprofloxacin. In this regard, Cifran ST should be administered 1-2 hours before or 4 hours after taking these drugs. This restriction does not apply to the class of H2-receptor blockers.
Calcium in food has little effect on absorption. However, simultaneous administration of ciprofloxacin and dairy or mineral-fortified products (such as milk, yoghurt, calcium-fortified orange juice) should be avoided, as absorption of ciprofloxacin may be reduced.
The combined use of Cifran ST and drugs containing theophylline may lead to an undesirable increase in the concentration of theophylline in the blood plasma, which, in turn, may cause the development of side effects. In rare cases, such side effects can be fatal. If the simultaneous use of these drugs cannot be avoided, the concentration of theophylline in the blood serum should be monitored and its dose should be reduced appropriately.
After concomitant use of Cifran ST and agents containing caffeine or pentoxifylline (oxpentifylline), an increase in the concentration of these xanthines in the blood serum has been reported.
The combined use of very high doses of quinolones (gyrase inhibitors) and some nonsteroidal anti-inflammatory drugs (excluding acetylsalicylic acid) may provoke seizures.
With the simultaneous use of Cifran ST and cyclosporine, in some cases an increase in serum creatinine concentration was observed, therefore such patients require frequent monitoring of this indicator (twice a week).
The anticoagulant effect of ciprofloxacin may be enhanced when Cifran ST is used concomitantly with a vitamin K antagonist. The risk may vary depending on the infection, age, and general condition of the patient, so it is difficult to assess the exact effect of ciprofloxacin on the increase in the international normalized ratio (INR). Frequent monitoring of INR should be performed during and immediately after the concomitant use of Cifran ST and a vitamin K antagonist (e.g. warfarin, acenocoumarol, phenprocoumon, or fluindione).
Due to the interaction of ciprofloxacin and glibenclamide, the effect of the latter may be enhanced, manifested by hypoglycemia.
The combined use of Cifran ST and probenecid is accompanied by an increase in the concentration of ciprofloxacin in the blood plasma.
When administered simultaneously with ciprofloxacin, a slowdown in tubular transport (renal metabolism) of methotrexate may occur, which may be accompanied by an increase in the concentration of methotrexate in the blood plasma. This increases the likelihood of side effects caused by methotrexate. The simultaneous administration of ciprofloxacin and methotrexate is not recommended.
Metoclopramide accelerates the absorption of ciprofloxacin, resulting in a shortened period of reaching the maximum concentration of ciprofloxacin in the blood plasma (this does not affect the bioavailability of the latter).
In a clinical study in healthy volunteers, concomitant use of ciprofloxacin and tizanidine resulted in an increase in plasma tizanidine concentrations (7-fold increase in Cmax, range: 4-21-fold; 10-fold increase in area under the concentration-time curve (AUC), range: 6-24-fold). Hypotensive and sedative side effects are associated with increased serum tizanidine concentrations. Therefore, concomitant use of ciprofloxacin and tizanidine is contraindicated.
In clinical studies, it was found that concomitant use of duloxetine and potent inhibitors of the CYP450 1A2 isoenzyme (such as fluvoxamine) may lead to an increase in duloxetine AUC and Cmax. Although there are no clinical data on the interaction with ciprofloxacin, the possibility of an interaction can be predicted when ciprofloxacin and duloxetine are used simultaneously.
Ciprofloxacin can be used in combination with azlocillin and ceftazidime for infections caused by Pseudomonas; with mezlocillin, azlocillin and other effective beta-lactam antibiotics for streptococcal infections; with isoxazole penicillins, vancomycin for staphylococcal infections; with metronidazole, clindamycin for anaerobic infections.
Uricosuric drugs (allopurinol) help slow down the elimination of ciprofloxacin by 50% and increase its concentration in blood plasma.
Co-administration of ropinirole with ciprofloxacin, a moderate inhibitor of the CYP450 1A2 isoenzyme, results in an increase in ropinirole AUC and Cmax by 60% and 84%, respectively.
Monitoring of ropinirole side effects and appropriate dose adjustment is recommended during and immediately after co-administration with ciprofloxacin.
Concomitant use of lidocaine-containing medicinal products and ciprofloxacin hydrochloride, a moderate inhibitor of the CYP450 1A2 isoenzyme, reduces the clearance of intravenously administered lidocaine by 22%. Although treatment with lidocaine was well tolerated, some interaction may occur after concomitant use with ciprofloxacin, which may be accompanied by adverse reactions.
After concomitant administration of 250 mg ciprofloxacin with clozapine for 7 days, serum concentrations of clozapine and N-desmethylclozapine were increased by 29% and 31%, respectively. Clinical monitoring and appropriate dose adjustment of clozapine is recommended during and immediately after co-administration with ciprofloxacin (see section 4.4).
Studies in healthy volunteers have shown an increase in Cmax and AUC of sildenafil by approximately twofold after oral administration of 50 mg with 500 mg of ciprofloxacin. Therefore, caution should be exercised when prescribing ciprofloxacin with sildenafil, carefully weighing the risks and benefits.
Simultaneous consumption of alcohol and the use of Cifran ST (due to the presence of tinidazole) may cause a disulfiram-like reaction, so alcohol should be excluded.
Anticoagulants: Drugs with similar chemical structures may potentiate the effects of oral anticoagulants. Prothrombin time should be monitored closely and the anticoagulant dose may need to be adjusted.
In clinical studies, fluvoxamine, a strong inhibitor of the CYP450 1A2 isoenzyme, has been shown to significantly inhibit the metabolism of agomelatine, resulting in a 60-fold increase in agomelatine exposure. Although there are no clinical data on a possible interaction with ciprofloxacin, a moderate inhibitor of CYP450 1A2, similar effects can be expected with concomitant administration.
Concomitant use of zolpidem and ciprofloxacin may increase zolpidem blood levels and is therefore not recommended.
Application features
The drug should be avoided in patients who have had serious adverse reactions to quinolones or fluoroquinolones in the past. Treatment of these patients with ciprofloxacin should only be initiated if there are no alternative treatment options and after a careful benefit/risk assessment.
Patients with epilepsy with a history of seizures, with vascular diseases and organic brain lesions, due to the risk of developing adverse reactions from the central nervous system, Cifran ST should be prescribed only for vital indications. If severe or prolonged diarrhea occurs during or after treatment with the drug, the possibility of developing pseudomembranous colitis should be excluded, which requires immediate discontinuation of the drug and the appointment of appropriate therapy.
Prolonged, disabling and potentially irreversible serious adverse reactions
In very rare cases, prolonged (months or years), disabling and potentially irreversible serious adverse reactions affecting various body systems, and sometimes several systems at once (musculoskeletal, nervous, mental and sensory), have been reported in patients treated with quinolones and fluoroquinolones, regardless of age and existing risk factors. The drug should be discontinued immediately at the first signs or symptoms of any serious adverse reaction and a doctor should be consulted.
Genital tract infections.
If genital tract infections are suspected or known to be caused by fluoroquinolone-resistant microorganisms, it is particularly important to obtain information on the extent of resistance to ciprofloxacin and tinidazole and to confirm susceptibility to the drug based on laboratory results.
Urinary tract infections.
Fluoroquinolone resistance in Escherichia coli, the most common pathogen causing urinary tract infections, varies across the European Union. Physicians are advised to consider the local prevalence of fluoroquinolone resistance in Escherichia coli when prescribing therapy.
Glucose-6-phosphate dehydrogenase deficiency.
Hemolytic reactions have been reported with ciprofloxacin in patients with glucose-6-phosphate dehydrogenase deficiency. Ciprofloxacin should be avoided in such patients unless the potential benefit outweighs the potential risk. In such cases, hemolysis should be observed.
Resistance.
During or after a course of treatment with ciprofloxacin, resistant bacteria may be isolated, with or without clinically apparent superinfection. There is a certain risk of isolation of ciprofloxacin-resistant bacteria during long courses of treatment and in the treatment of nosocomial infections and/or infections caused by Staphylococcus and Pseudomonas species.
Methotrexate.
The simultaneous use of ciprofloxacin and methotrexate is not recommended (see section "Interaction with other medicinal products and other types of interactions").
Cardiac disorders.
Fluoroquinolones, including ciprofloxacin, should be used with caution in patients with known risk factors for QT prolongation, including:
with hereditary QT prolongation syndrome; in case of simultaneous use of drugs that can prolong the QT interval (e.g., antiarrhythmics of classes IA and III, tricyclic antidepressants, macrolides, neuroleptics); in case of uncorrected electrolyte imbalance (e.g., hypokalemia, hypomagnesemia); in case of heart disease (e.g., heart failure, myocardial infarction, bradycardia).
Elderly patients and women may be more sensitive to drugs that prolong the QTc interval. Therefore, fluoroquinolones, including ciprofloxacin, should be used with caution in these patient groups (see sections 4.2, 4.8, 4.8, and 4.9).
Aortic aneurysm and dissection
Therefore, fluoroquinolones should only be used after careful benefit/risk assessment and after consideration of other treatment options in patients with a positive family history of aneurysm or in patients with a diagnosis of aortic aneurysm and/or aortic dissection, or in the presence of risk factors or conditions predisposing to aortic aneurysm and dissection (e.g. Marfan syndrome, Ehlers-Danlos vascular syndrome, Takayasu arteritis, giant cell arteritis, Behçet's disease, arterial hypertension, known atherosclerosis).
In case of sudden abdominal, chest or back pain, patients should be advised to seek immediate medical attention in the emergency department.
Dysglycemia.
As with other quinolones, glucose disturbances, both hypoglycemia and hyperglycemia, have occurred in diabetic patients receiving oral antidiabetic agents (e.g. glibenclamide) or insulin. These reactions were more common in elderly patients. Cases of hypoglycemic coma have been reported. Close monitoring of blood glucose levels is recommended in all diabetic patients (see section 4.8).
Digestive tract.
The occurrence of severe and persistent diarrhea during and after treatment may be a manifestation of a serious gastrointestinal disease (e.g. life-threatening pseudomembranous colitis) that requires immediate treatment. In such cases, the drug should be discontinued and appropriate therapy should be initiated. Drugs that inhibit peristalsis are contraindicated.
Transient increases in transaminases, alkaline phosphatase or cholestatic jaundice may occur, especially in patients with a history of liver disease.
Kidneys and urinary system.
Crystalluria has been reported in association with the use of ciprofloxacin (see section 4.8). Patients receiving ciprofloxacin should be adequately hydrated. Excessive alkalinity of the urine should be avoided.
Kidney dysfunction.
Since ciprofloxacin is excreted mainly unchanged by the kidneys, the dose should be adjusted in patients with impaired renal function as described in the section "Method of administration and dosage" to avoid an increase in the frequency of adverse reactions caused by accumulation of ciprofloxacin.
Hepatobiliary system.
Cases of hepatic necrosis and life-threatening hepatic failure have been reported with ciprofloxacin (see section 4.8). Treatment should be discontinued if any symptoms of liver disease (such as anorexia, jaundice, dark urine, pruritus or abdominal tenderness) occur.
Nervous system.
Patients with epilepsy and patients with a history of central nervous system dysfunction (e.g., decreased seizure threshold, seizures, decreased cerebral circulation, organic brain damage, and stroke) should only use the drug if the expected benefit outweighs the potential risk. In some cases, adverse reactions from the central nervous system are observed after the first dose of the drug. In rare cases, depression or psychosis may progress. In such cases, the drug should be discontinued.
Peripheral neuropathy
Cases of sensory or sensorimotor polyneuropathy resulting in paresthesia, hypoesthesia, dysesthesia, or weakness have been reported in patients treated with quinolones and fluoroquinolones. If symptoms of neuropathy such as pain, burning, tingling, numbness, or weakness occur, patients taking the drug should inform their physician to prevent the development of a potentially irreversible condition.
Tinidazole in Cifran ST has occasionally caused various neurological disorders, such as dizziness, ataxia, peripheral neuropathies, and only rarely - seizures. In the event of any neurological disorders, the drug should be discontinued.
Hypersensitivity to the drug.
In some cases, hypersensitivity and allergic reactions are observed after the first dose of the drug. Very rarely, anaphylactic/anaphylactoid reactions may occur, up to shock, which threatens the patient's life. In some cases, they are observed after the first dose of ciprofloxacin. In such cases, the drug should be discontinued and medical treatment should be initiated immediately.
Musculoskeletal system.
In general, ciprofloxacin should not be used in patients with tendon diseases or a history of quinolone-related disorders. However, in rare cases, after microbiological investigation of the pathogen and an assessment of the benefit/risk ratio, ciprofloxacin may be prescribed to these patients for the treatment of certain severe infections, namely in cases of failure of standard therapy or bacterial resistance, when the results of microbiological studies justify the use of ciprofloxacin.
In general, ciprofloxacin should not be used in patients with a history of tendon disease or disorders associated with the use of quinolones. However, in rare cases, after microbiological investigation of the pathogen and an assessment of the benefit/risk ratio, ciprofloxacin may be prescribed to these patients for the treatment of certain severe infections, namely in cases of failure of standard therapy or bacterial resistance, when microbiological results justify the use of ciprofloxacin. Tendinitis and tendon rupture (not limited to the Achilles tendon), sometimes bilateral, may occur as early as 48 hours after the start of treatment with quinolones and fluoroquinolones and even up to several months after discontinuation of treatment. The risk of tendonitis and tendon rupture is increased in elderly patients, patients with impaired renal function, patients with solid organ transplantation and patients receiving concomitant corticosteroids. Therefore, concomitant use of corticosteroids should be avoided.
At the first sign of tendinitis (e.g. painful swelling, inflammation), treatment with the drug should be discontinued and alternative treatment should be considered. The affected limb(s) should be treated appropriately (e.g. immobilization). Corticosteroids should not be used if signs of tendinopathy occur.
Vision disorders
If you experience decreased vision or any effect on your eyes, you should consult a doctor.
Skin.
Ciprofloxacin causes photosensitivity reactions. Patients taking the drug should avoid intense ultraviolet radiation. If photosensitivity reactions (e.g., sunburn-like) occur, therapy with the drug should be discontinued.
Cytochrome P 450.
Ciprofloxacin is known to be a moderate inhibitor of cytochrome P450 1A2 enzymes. Caution should be exercised when using ciprofloxacin and drugs metabolized by these enzymes, such as theophylline, methylxanthine, caffeine, duloxetine, clozapine, etc., since an increase in the serum concentration of these drugs may cause specific side effects. During treatment and for at least 72 hours after treatment with Cifran ST (due to the presence of tinidazole), alcoholic beverages should be avoided, taking into account the possibility of an Antabuse-like reaction (hot flashes, colicky abdominal pain, tachycardia).
Impact on laboratory test results
Ciprofloxacin may interfere with culture results for Mycobacterium spp. in vitro by inhibiting the growth of mycobacterial cultures, which may lead to false-negative culture results in patients taking ciprofloxacin.
During treatment with the drug, changes in some laboratory parameters are possible: the appearance of sediment in the urine; temporary increase in the concentration of urea, creatinine, bilirubin, hepatic transaminases in the blood serum; in some cases - hyperglycemia, crystalluria or hematuria, changes in prothrombin parameters. In patients with impaired liver and/or kidney function, it is recommended to monitor the concentration of ciprofloxacin in the blood plasma.
The drug is not recommended for use in patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption.
The simultaneous intake of tablets and dairy or calcium-fortified products (e.g. milk, yoghurt, juices with a high calcium content) should be avoided. Other calcium-containing products do not affect the absorption of ciprofloxacin.
The drug is not indicated for the treatment of acute tonsillitis (tonsillar angina).
Ability to influence reaction speed when driving vehicles or other mechanisms
Patients taking Cifran ST should refrain from activities requiring increased attention and speed of psychomotor reactions, as well as from driving vehicles.
Use during pregnancy or breastfeeding
Cifran ST should not be prescribed to pregnant and breastfeeding women.
Given the data from animal studies, the possibility of damage to articular cartilage in newborns cannot be completely ruled out, while the possibility of teratogenic effects (malformations) has not been confirmed.
Tinidazole passes into breast milk and is detected for at least 72 hours after administration. Women should not breastfeed while taking the drug and for at least 3 days after stopping the drug.
Method of administration and doses
Cifran ST should be taken orally with sufficient water. The dosage regimen and duration of treatment are determined by the doctor individually depending on the localization, severity of the pathological process, and the sensitivity of the pathogens.
Adults are recommended to take 1 tablet 2 times a day.
The maximum daily dose is 2 tablets of Cifran ST.
Elderly patients should receive a dose depending on the severity of the infection and the patient's creatinine clearance. For patients with creatinine clearance from 31 to 60 ml/min, the maximum daily dose of the drug should be 2 tablets per day, for patients with creatinine clearance 30 ml/min or less - 1 tablet per day.
The course of treatment for acute uncomplicated infections is from 1 to 7 days, for the treatment of complicated and chronic recurrent infections - 10-14 days. For infections caused by streptococci, treatment should be continued for at least 10 days to avoid the risk of developing complications in the future. For infections caused by Chlamydia, the course of treatment should also last at least 10 days. For osteomyelitis, the course of treatment can be up to 2 months. In patients with reduced immunity, treatment should be carried out throughout the entire period of neutropenia. Taking Cifran ST should be continued for 2 days after the symptoms of the disease disappear.
Children
Do not use on children.
Overdose
In some cases, reversible renal parenchymal toxicity has been observed following oral ciprofloxacin overdose. Therefore, in the event of overdose, in addition to standard measures (gastric lavage, induction of vomiting, administration of large amounts of fluid, acidification of urine), it is recommended to monitor renal function and administer antacids containing magnesium and calcium, which reduce the absorption of ciprofloxacin. Only a small amount of ciprofloxacin (< 10%) is removed by hemodialysis.
Specific antidote is unknown.
Non-serious cases of overdose have been reported in patients taking tinidazole, but they do not provide a complete picture of the symptoms of overdose.
There is no specific antidote for tinidazole overdose. Treatment should be symptomatic and supportive. Gastric lavage may be useful. Tinidazole is readily removed by hemodialysis.
Adverse reactions
Caused by ciprofloxacin.
Infections and infestations:
candidiasis – infrequently; antibiotic-associated colitis – rarely, very rarely – fatal.
From the hematopoietic and lymphatic systems:
eosinophilia - uncommon; leukopenia, anemia, neutropenia, leukocytosis, thrombocytopenia, thrombocytosis - rare; hemolytic anemia, agranulocytosis, pancytopenia (life-threatening), bone marrow depression (life-threatening) - very rare.
On the part of the immune system:
Allergic reactions, allergic/angioedema - rare; anaphylactoid reactions, anaphylactic shock (life-threatening) and serum sickness-like reactions - very rare.
Mental disorders*:
psychomotor agitation/anxiety - uncommon; confusion and disorientation, restlessness, increased drowsiness, depression (which may lead to suicidal thoughts and behavior), hallucinations - rare; psychosis - very rare.
Mania, including hypomania - frequency not established.
Nervous system*:
headache, dizziness, sleep disorders, taste disturbances - uncommon; paresthesia, dysesthesia, hypoesthesia, tremor, convulsions, vertigo - rare; migraine, coordination disorders, olfactory disorders, hyperesthesia and intracranial hypertension - very rare; peripheral neuropathy and polyneuropathy - frequency unknown.
From the organs of vision*:
Visual disturbances (e.g. diplopia, visual anomalies, chromatopsia) are rare; colour vision disturbances are very rare.
Sensory and labyrinth disorders*:
Ringing in the ears, deafness – rare; hearing impairment – very rare.
From the cardiovascular system:
tachycardia - rare; vasodilation, decreased blood pressure, fainting - rare; vasculitis - very rare; QT prolongation, ventricular arrhythmia, bidirectional ventricular tachycardia - frequency unknown.
On the part of the respiratory system:
dyspnea (including asthmatic conditions) – rare.
From the gastrointestinal tract:
anorexia - uncommon; nausea, diarrhea - common; vomiting, bitter taste in the mouth, epigastric pain, dyspeptic disorders, flatulence, antibiotic-associated colitis (very rare - fatal) - uncommon; pancreatitis - very rare.
From the endocrine system: hyperglycemia, hypoglycemia (cases of hypoglycemic coma);
syndrome of inappropriate antidiuretic hormone secretion;
From the liver and biliary tract:
Transient increase in transaminases, hyperbilirubinemia - uncommon; liver function abnormalities, jaundice, hepatitis (non-infectious) - rare; liver necrosis (which very rarely progresses to life-threatening liver failure) - very rare.
From the skin and subcutaneous tissue:
rashes (petechial, macular, urticaria, etc.), itching, urticaria - infrequently; photosensitivity reactions, the appearance of nonspecific blisters - rarely; petechiae, erythema multiforme, erythema nodosum, Stevens-Johnson syndrome and toxic epidermal necrolysis - very rarely.
Acute generalized exanthematous pustulosis (AGEP), DRESS syndrome with eosinophilia and systemic symptoms – frequency not established.
Musculoskeletal and connective tissue disorders*:
arthralgias - infrequently; myalgias, arthritis, increased muscle tone and cramps - rarely; muscular weakness, tendinitis, tendon ruptures (mainly Achilles), exacerbation of myasthenia gravis symptoms - very rarely.
From the urinary system:
renal dysfunction - uncommon; tubulointerstitial nephritis, renal failure, hematuria, crystalluria - rare.
From the body
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.