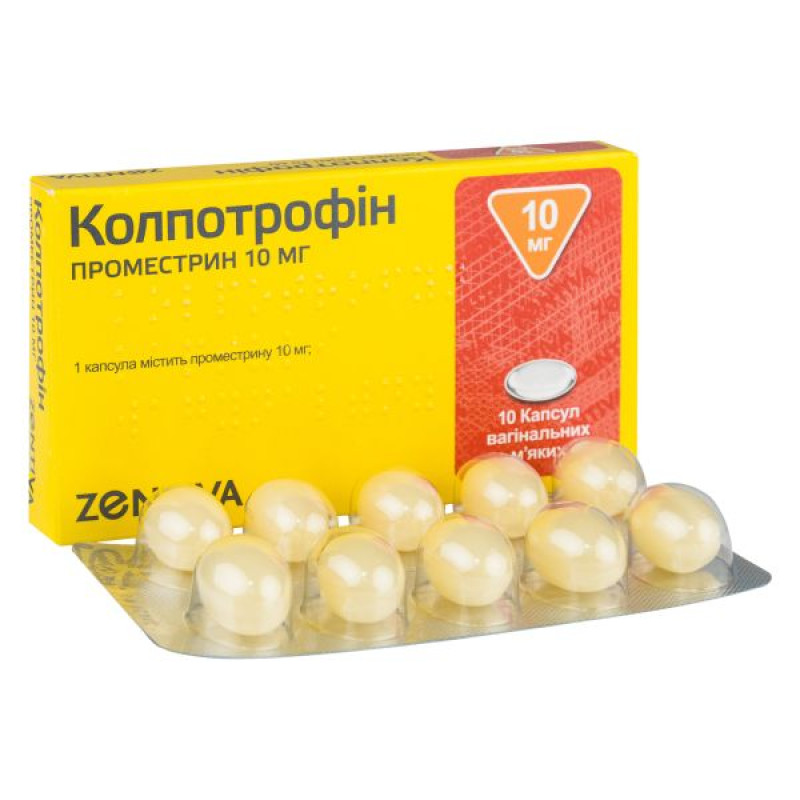
Colpotrophin vaginal soft capsules 10 mg blister No. 10

Instructions for Colpotrophin soft vaginal capsules 10 mg blister No. 10
Composition
active ingredient: promestrin;
1 capsule contains 10 mg of promestrin;
excipients: sodium methylparaben (E 219), sodium propylparaben (E 217), white soft paraffin, perhydrogenated polyisobutene, sorbitan sesquioleate, colloidal anhydrous silicon dioxide, purified water, gelatin, glycerin, dimethicone.
Dosage form
Soft vaginal capsules.
Main physicochemical properties: oval soft capsules of pale yellow color.
Pharmacotherapeutic group
Simple preparations of natural and semi-synthetic estrogens. ATX code G03C A09.
Pharmacological properties
Pharmacodynamics.
Promestrin is a synthetic estrogen-like agent intended for topical use, which has a local estrogenic effect on the mucous membrane of the lower parts of the urogenital tract, restoring their trophism. When administered intravaginally, it does not have a systemic effect, therefore it does not affect the endometrium, mammary glands and pituitary gland.
Promestrine exerts a local estrogenic effect on the vaginal mucosa: it promotes the proliferation of the vaginal epithelium.
Pharmacokinetics.
When used intravaginally, the tablet interacts with the vaginal secretion, splits and its components are released. Promestrine does not accumulate in tissues, less than 1% of promestrin is absorbed, its half-life is 24 hours. When administered intravaginally, promestrin does not have a resorptive effect, and there are no systemic hormonal effects.
Indication
Vaginal atrophy caused by estrogen deficiency. Delayed healing of the vagina, cervix, and vulva after childbirth, surgery, or physiotherapy.
Contraindication
Hypersensitivity to any component of the drug.
Despite the absence of systemic effects when using Colpotrophin, this drug should not be used, like any estrogens, for estrogen-dependent tumors (such as breast cancer, endometrium).
The drug should not be used simultaneously with spermicides or latex condoms.
Interaction with other medicinal products and other types of interactions
Colpotrophin can inactivate the effect of any spermicidal agents, so their simultaneous use is not recommended. The drug should not be used while using a latex condom, since oily substances and lubricants containing mineral oils reduce its strength and increase the likelihood of rupture.
Application features
During treatment, you should be under the supervision of a doctor.
If metrorrhagia occurs, it is necessary to determine the cause and provide supportive treatment.
Despite its oiliness, the liquid contained in the capsules is an emulsion and is easily washed off with water.
Unless otherwise prescribed by a doctor, daily vaginal douching is not required; external washing with soap and water is sufficient.
In some cases, it may be appropriate to use sanitary napkins.
The drug contains sodium methylparaben (E 219) and sodium propylparaben.
(E 217). May cause allergic reactions (sometimes delayed).
Use during pregnancy or breastfeeding
Colpotrophin is not indicated for use during pregnancy.
However, the results of numerous epidemiological studies show that, unlike diethylstilbestrol, the use of estrogens in early pregnancy is not associated with a risk of malformations. Thus, if pregnancy occurs during use of the drug, there is no need to terminate it.
When using a condom, there is a risk of it breaking, as oily substances and lubricants containing mineral oils reduce its strength.
Given the lack of sufficient data on the penetration of the drug into breast milk, its use should be avoided during breastfeeding.
Ability to influence reaction speed when driving vehicles or other mechanisms
Does not affect.
Method of administration and doses
1 capsule (pre-moistened) is inserted deep into the vagina for 20 days. If necessary, treatment can be extended (menopause, castration, use of estrogen-progestin-containing contraceptives).
Children
Not used.
Overdose
Given the route of administration and the low absorption of promestrin, systemic overdose is unlikely.
However, overdose may be accompanied by increased local adverse reactions (irritation, itching, burning) in the vulva area.
Adverse reactions
Skin and subcutaneous tissue disorders
Very rare: local reactions including irritation, itching, burning.
On the part of the immune system
Very rare: allergic reactions.
Expiration date
5 years.
Storage conditions
Store at a temperature not exceeding 25 °C out of the reach of children.
Packaging
10 capsules in a blister, 1 blister in a cardboard box.
Vacation category
According to the recipe.
Producer
Location of the manufacturer and address of its place of business
St. Svensweg 5, 2031 GA Haarlem, Netherlands.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.