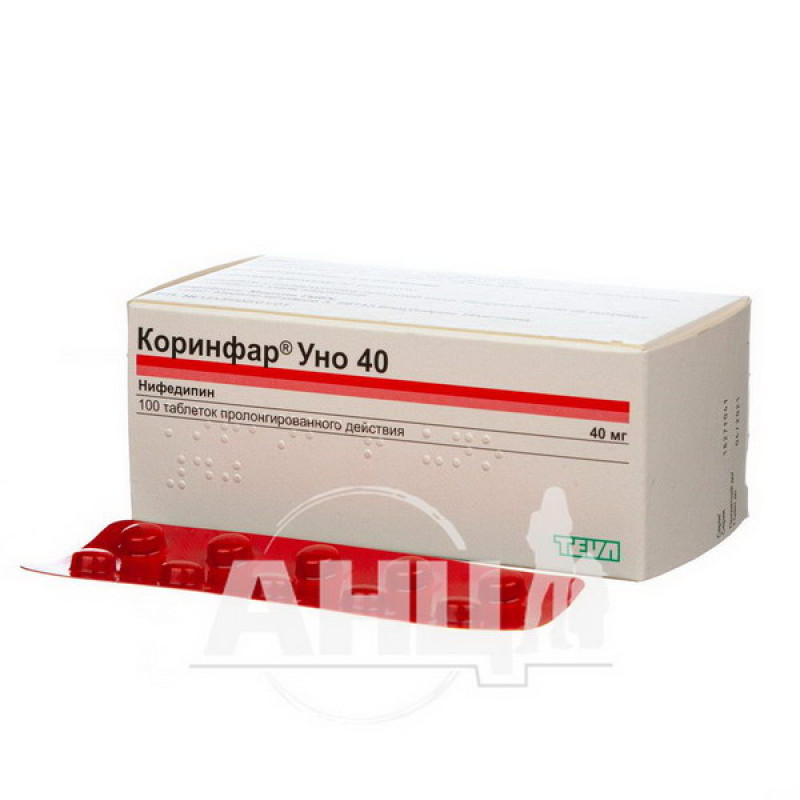
Corinfar uno 40 prolonged-release film-coated tablets 40 mg blister No. 100
Instructions Corinfar uno 40 prolonged-release film-coated tablets 40 mg blister No. 100
Composition
active ingredient: nifedipine;
1 tablet contains nifedipine 40 mg;
excipients: microcrystalline cellulose, cellulose, lactose monohydrate, magnesium stearate, hypromellose, colloidal anhydrous silica, macrogol, talc, red iron oxide (E 172), titanium dioxide (E 171).
Dosage form
Extended-release tablets.
Main physicochemical properties: red-brown round biconvex tablets with a diameter of 7 mm, film-coated.
Pharmacotherapeutic group
Selective calcium antagonists with a predominant effect on blood vessels. ATX code C08C A05.
Pharmacological properties
Pharmacodynamics
Nifedipine is a calcium antagonist of the 1,4-dihydropyridine type. Calcium antagonists reduce the influx of calcium ions through "slow" calcium channels into cells. Nifedipine acts mainly on the smooth muscles of the coronary arteries and peripheral vessels, with virtually no effect on the myocardium when used in therapeutic doses.
Nifedipine reduces peripheral vascular resistance (afterload) and promotes coronary artery dilation, which leads to improved blood circulation.
At the beginning of therapy with calcium antagonists, a reflex increase in heart rate and cardiac output may be observed. However, this is not sufficient to compensate for the vasodilation.
In cases of long-term therapy with nifedipine, the initial increase in cardiac output returns to previous values. A significant decrease in blood pressure against the background of nifedipine use may be observed in patients with arterial hypertension.
Pharmacokinetics
After oral administration on an empty stomach, nifedipine is rapidly and almost completely absorbed. Nifedipine has a "first pass" effect through the liver, the systemic bioavailability of the drug when taken orally is 50-70%. The maximum concentration of nifedipine in blood plasma and serum is reached after approximately 30-85 minutes - when taking the drug in the form of prolonged-release tablets. 95-98% of nifedipine binds to blood plasma proteins (albumin). The average volume of distribution of nifedipine (Vss) is 0.77-1.12 l/kg. After oral administration, nifedipine is almost completely metabolized in the liver, mainly due to oxidative processes, the metabolites are inactive.
The drug is almost completely eliminated from the body within 24 hours. Nifedipine is excreted mainly by the kidneys as metabolites and only 5-15% with bile in the feces. Only trace amounts of unchanged substance (less than 0.1%) were detected in the urine.
The duration of the half-life is 10 hours.
Accumulation of the drug in the body during long-term treatment with therapeutic doses has not been described. With reduced liver function, a clear prolongation of the half-life of the active substance and a decrease in total plasma clearance are observed. If necessary, the dose of the drug should be reduced in such cases.
Indication
Essential hypertension.
Contraindication
Hypersensitivity to the active substance or to any component of the drug; cardiogenic shock; unstable angina; acute myocardial infarction (within the first 4 weeks); acute angina attack; secondary prevention of myocardial infarction; malignant hypertension (safety of the drug has not been studied); high-grade aortic stenosis; ileostomy or colostomy; concomitant use of rifampicin (due to the inability to achieve effective levels of nifedipine in the blood plasma due to enzyme induction); pregnancy.
Interaction with other medicinal products and other types of interactions
Drugs that affect the effectiveness of nifedipine
Nifedipine is metabolized via the cytochrome P450 3A4 system, therefore drugs that inhibit or induce this enzyme system may alter the "first pass" (after oral administration) or clearance of nifedipine.
When using nifedipine together with the following drugs, the degree and duration of interaction should be taken into account.
Rifampicin
Rifampicin significantly induces the cytochrome P450 3A4 system. When used simultaneously with rifampicin, the bioavailability of nifedipine is significantly reduced, and thus its effectiveness is weakened. In view of this, the use of the combination of nifedipine with rifampicin is contraindicated.
When using the following weak or moderate inhibitors of the cytochrome P450 3A4 system simultaneously, blood pressure should be monitored and, if necessary, the dose of nifedipine should be reduced.
Macrolide antibiotics (e.g. erythromycin)
No interaction studies have been conducted between nifedipine and macrolide antibiotics. Some macrolide antibiotics inhibit the cytochrome P450 3A4-mediated metabolism of other drugs. Therefore, the possibility of an increase in nifedipine plasma concentrations cannot be excluded when both drugs are administered simultaneously.
Azithromycin, which is structurally similar to members of the macrolide class of antibiotics, does not inhibit CYP3A4.
Interaction studies between nifedipine and certain anti-HIV protease inhibitors have not been conducted. Drugs of this class are known to inhibit the cytochrome P450 3A4 system. In addition, drugs of this class inhibit the cytochrome P450 3A4-mediated metabolism of nifedipine in vitro. When used simultaneously with nifedipine, a significant increase in nifedipine plasma concentrations due to a decrease in first-pass metabolism and a decrease in the rate of elimination cannot be excluded.
Azole antifungals (e.g. ketoconazole)
No formal clinical study has been conducted on the interaction of nifedipine with certain azole antifungals. Drugs of this class are known to inhibit the cytochrome P450 3A4 system. When administered orally with nifedipine, a significant increase in the systemic bioavailability of nifedipine due to a decrease in first-pass metabolism cannot be excluded.
Fluoxetine
Interaction studies between nifedipine and fluoxetine have not been conducted. Fluoxetine is known to inhibit the cytochrome P450 3A4-mediated metabolism of nifedipine in vitro. An increase in nifedipine plasma concentrations cannot be excluded when both drugs are used simultaneously.
Nefazodone
Interaction studies between nifedipine and nefazodone have not been conducted. Nefazodone is known to inhibit in vitro cytochrome P450 3A4-mediated metabolism of other drugs. When both drugs are used simultaneously, an increase in nifedipine plasma concentrations cannot be excluded.
Quinupristin/dalfopristin
Concomitant use of quinupristin/dalfopristin and nifedipine may lead to an increase in nifedipine plasma concentrations.
Valproic acid
Interaction studies of nifedipine and valproic acid have not been conducted. Valproic acid is known to increase plasma concentrations of the structurally similar calcium channel blocker nimodipine due to enzyme inhibition. Therefore, an increase in nifedipine plasma concentrations and efficacy cannot be excluded.
Cimetidine
Due to inhibition of cytochrome P450 3A4, cimetidine increases nifedipine plasma concentrations and may enhance the antihypertensive effect.
Tricyclic antidepressants, vasodilators
In the case of combining nifedipine with tricyclic antidepressants and vasodilators, there is a possible increase in the hypotensive effect.
Additional research
Cisapride
Concomitant use of cisapride and nifedipine may lead to an increase in the concentration of nifedipine in the blood plasma.
Antiepileptic drugs that induce the cytochrome P450 3A4 system, such as phenytoin, carbamazepine, and phenobarbital
Phenytoin induces the cytochrome P450 3A4 system. When used simultaneously with phenytoin, the bioavailability of nifedipine is reduced and the efficacy is weakened. When both drugs are used simultaneously, the clinical response to nifedipine therapy should be monitored and, if necessary, an increase in the nifedipine dose should be considered. If the nifedipine dose has been increased during the simultaneous use of both drugs, a decrease in the nifedipine dose should be considered when phenytoin is discontinued.
Formal clinical studies on the interaction of nifedipine with carbamazepine or phenobarbital have not been conducted. Both drugs are known to reduce plasma concentrations of the structurally similar calcium channel blocker nimodipine due to enzyme induction. Therefore, a decrease in nifedipine plasma concentrations and reduced efficacy cannot be excluded.
Diltiazem reduces the breakdown of nifedipine, which may require a dose reduction.
Effect of nifedipine on other drugs
Antihypertensive drugs
Nifedipine may increase the antihypertensive effect of concomitantly used antihypertensive drugs, such as:
diuretics; β-adrenergic blockers; ACE (angiotensin-converting enzyme) inhibitors; AT1 receptor antagonists; other calcium antagonists; α-adrenergic blockers; PDE-5 (phosphodiesterase-5) inhibitors; α-methyldopa; magnesium sulfate.
When nifedipine is used concomitantly with β-adrenergic blockers, careful monitoring of the patient's condition is required, as isolated cases of exacerbation of heart failure are known.
Nitrates
Simultaneous use of the drug with nitrates leads to an increased effect on blood pressure and heart rate.
Digoxin, theophylline
Nifedipine causes an increase in plasma levels of digoxin and theophylline (consider symptoms of digoxin overdose, after determining the digoxin level, a reduction in the dose of the glycoside may be necessary). The patient's condition should be closely monitored.
Quinidine
Therefore, blood pressure should be carefully monitored when quinidine is included in the nifedipine regimen. If necessary, the nifedipine dose should be reduced.
Tacrolimus
Tacrolimus is known to be metabolised by the cytochrome P450 3A4 system. Published data suggest that in some cases the dose of tacrolimus may need to be reduced when co-administered with nifedipine. Plasma tacrolimus concentrations should be monitored when both drugs are co-administered and a reduction in the tacrolimus dose should be considered if necessary.
With simultaneous use of vincristine, there is a weakening of the excretion of vincristine, therefore, adverse reactions of vincristine may increase - the need for a dose reduction should be considered. In the case of simultaneous use of cephalosporins, there is an increase in cephalosporin levels in the blood plasma.
Interaction with food products
Grapefruit juice
Grapefruit juice inhibits the cytochrome P450 3A4 system. The use of grapefruit juice while taking nifedipine leads to an increase in the concentration of the drug in the blood plasma and an increase in the duration of action of nifedipine due to a decrease in first-pass metabolism or a decrease in clearance. As a result, the antihypertensive effect of the drug may be enhanced. After regular consumption of grapefruit juice, this effect may persist for at least 3 days after the last intake of the juice.
Therefore, grapefruit/grapefruit juice should be avoided during nifedipine therapy.
Pharmacokinetic interactions
Aymalin. Simultaneous use of Aymalin and nifedipine does not affect the pharmacokinetics of nifedipine.
Acetylsalicylic acid. The use of 100 mg of acetylsalicylic acid does not affect the pharmacokinetics of nifedipine. Simultaneous use with nifedipine does not change the effect of acetylsalicylic acid on platelet aggregation and bleeding time.
Benazepril: Concomitant administration of benazepril and nifedipine does not affect the pharmacokinetics of nifedipine.
Debrisoquine: Concomitant administration of nifedipine and debrisoquine does not affect the pharmacokinetics of nifedipine.
Talinolol: Concomitant administration of nifedipine and talinolol does not affect the pharmacokinetics of nifedipine.
Irbesartan: Concomitant use of nifedipine and irbesartan does not affect the pharmacokinetics of irbesartan.
Candesartan cilexetil. Concomitant use of nifedipine and candesartan cilexetil does not affect the pharmacokinetics of either drug.
Orlistat: Concomitant administration of nifedipine and orlistat does not affect the pharmacokinetics of nifedipine.
Omeprazole: Concomitant administration of nifedipine and omeprazole does not affect the pharmacokinetics of nifedipine.
Pantoprazole: Concomitant use of nifedipine and pantoprazole does not affect the pharmacokinetics of nifedipine.
Ranitidine: Concomitant use of nifedipine and ranitidine does not affect the pharmacokinetics of nifedipine.
Rosiglitazone: Concomitant administration of nifedipine and rosiglitazone does not affect the pharmacokinetics of nifedipine.
Triamterene hydrochloride: Concomitant administration of nifedipine and triamterene hydrochloride does not affect the pharmacokinetics of nifedipine.
Other types of interaction
The use of nifedipine may lead to false-positive results in spectrophotometric determination of the concentration of vanillylmandelic acid in urine (however, this effect is not observed when using the high-performance liquid chromatography method).
Application features
The drug should be used with caution in cases of severe arterial hypotension (systolic blood pressure below 90 mm Hg) and severe heart failure.
There are no data on the safety and efficacy of the drug based on well-controlled studies involving pregnant women.
Animal studies have shown a range of embryotoxic, placentaltoxic and fetotoxic effects when administered during and after the period of organogenesis.
Based on the available clinical evidence, no specific prenatal risk has been identified. Although an increased incidence of perinatal asphyxia, cesarean section, preterm birth, and intrauterine growth retardation have been reported, it is not clear whether these events are due to the presence of hypertension, its treatment, or a specific effect of the drug.
The available information is insufficient to exclude serious adverse effects on the fetus or newborn. Therefore, any use of the drug during pregnancy requires a very careful risk-benefit assessment, and the question of drug therapy should be considered only if alternative treatment is not indicated or has proven ineffective.
When nifedipine is used concomitantly with intravenous magnesium sulfate, careful monitoring of blood pressure is required due to the possibility of a significant decrease in blood pressure, which could harm the mother and fetus.
Caution should be exercised when using the drug tablets if the patient has severe narrowing of the gastrointestinal tract - due to the possibility of obstructive symptoms. Very rarely, bezoars may occur, which may require surgical intervention.
The drug should not be used in patients with an ileostomy (after proctocolectomy).
The use of the medicinal product may lead to false-positive results in X-ray examinations using barium contrast media (e.g. filling defects interpreted as polyps).
Patients with impaired liver function require careful monitoring of the condition, and in severe cases, a dose reduction.
Nifedipine is metabolized via the cytochrome P450 3A4 system, therefore drugs that inhibit or induce this enzyme system may alter the "first pass" or clearance of nifedipine.
Drugs that are weak or moderate inhibitors of the cytochrome P450 3A4 system and may lead to an increase in nifedipine plasma concentrations include, for example:
macrolide antibiotics (e.g. erythromycin); anti-HIV protease inhibitors (e.g. ritonavir); azole antifungals (e.g. ketoconazole); antidepressants nefazodone and fluoxetine; quinupristin/dalfopristin; valproic acid; cimetidine.
When using the drug with these drugs, it is necessary to monitor blood pressure and, if necessary, consider reducing the dose of nifedipine.
Some in vitro experiments have shown a link between the use of calcium antagonists, in particular nifedipine, and reversible biochemical changes in spermatozoa, which impair their ability to fertilize. If in vitro fertilization attempts are unsuccessful, in the absence of other explanations, calcium antagonists, such as nifedipine, may be considered as a possible cause of this phenomenon.
The drug should not be used if there is a connection between previous use of nifedipine and ischemic pain. In patients with angina pectoris, the drug may provoke attacks, their duration and intensity may increase, especially at the beginning of treatment.
Medicines with the active ingredient nifedipine should not be used in patients with unstable angina.
The drug should be administered with particular caution to patients on hemodialysis, in conditions of malignant arterial hypertension or hypovolemia, since dilation of blood vessels may cause a significant decrease in blood pressure in them.
The use of nifedipine in patients with diabetes mellitus may require treatment adjustment.
The medicinal product contains lactose monohydrate. Patients suffering from rare hereditary diseases such as galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption should not be prescribed “Corinfar® Uno 40”.
Ability to influence reaction speed when driving vehicles or other mechanisms
Therapy with nifedipine requires constant medical supervision. Due to possible individual sensitivity, some patients may experience impaired ability to drive or operate machinery. These precautions mainly concern the initial period of therapy, the period of increasing the dose of the drug, switching to another drug, and alcohol consumption.
Use during pregnancy or breastfeeding
Pregnancy
The use of nifedipine is contraindicated during pregnancy.
The use of nifedipine during pregnancy requires a careful assessment of the risk/benefit ratio, and the question of therapy with the drug should be considered only when alternative treatment is not indicated or has proven ineffective. There are no data from studies of the use of the drug in pregnant women.
Animal studies have shown embryotoxicity, fetotoxicity, and teratogenicity of the drug.
Cases of perinatal asphyxia, complications of cesarean section, risk of preterm birth, and intrauterine growth retardation have been reported. It is currently unknown whether these problems are caused by essential hypertension, antihypertensive therapy, or the effects of a specific drug.
Acute pulmonary edema has been reported (especially in multiple pregnancies) when intravenous calcium channel blockers, including nifedipine, have been used to reduce labor and/or when β2-agonists have been used concomitantly.
When using the drug simultaneously with intravenous administration of magnesium sulfate, careful monitoring of blood pressure is necessary due to the possibility of a significant decrease in blood pressure, which may harm the mother and fetus.
Breast-feeding
Nifedipine passes into breast milk (the concentration of nifedipine in breast milk is almost the same as the concentration of nifedipine in the mother's blood plasma), therefore, if necessary, the use of nifedipine, breastfeeding should be discontinued.
Fertility
In isolated cases, an association has been found between calcium antagonists such as nifedipine and reversible biochemical changes occurring in the head of spermatozoa during in vitro fertilization, which may lead to impaired sperm function. If a repeat in vitro fertilization attempt is unsuccessful, in the absence of other explanations, this may be due to the effect of calcium antagonists such as nifedipine.
Method of administration and doses
Depending on the patient's condition, in each individual case the dose should be increased gradually until the optimal therapeutic effect is achieved.
Patients with severe cerebral circulation disorders (severe cerebrovascular disease) should be prescribed the drug in reduced doses.
Essential hypertension
The average daily dose is 1 tablet once a day (40 mg once a day).
Method of application
The drug should be taken orally in the morning 30 minutes before breakfast, without chewing and with sufficient liquid (for example, 1 glass of water). It is recommended to take the tablets at the same time. It is necessary to avoid drinking grapefruit juice with the drug.
Simultaneous food intake increases bioavailability and maximum plasma concentrations of the active substance.
“Corinfar® Uno 40” tablets cannot be divided, because then the protection from light provided by the tablet coating will not be guaranteed.
The duration of treatment is determined by the doctor.
Special patient groups
Children and adolescents: The safety and efficacy of nifedipine treatment in children (under 18 years of age) have not been established.
Elderly patients (>65 years): Based on pharmacokinetic parameters, no dose adjustment is necessary for patients over 65 years of age.
Hepatic impairment: Patients with hepatic impairment may require close monitoring and, in some cases, a dose reduction.
Renal impairment: Based on pharmacokinetic parameters, no dose adjustment is necessary for patients with renal impairment.
Children
The safety and efficacy of nifedipine in children (under 18 years of age) have not been established. The drug should not be used in children.
Overdose
Symptoms of acute intoxication: impaired consciousness, coma, hypotension, tachycardia/bradycardia, hyperglycemia, metabolic acidosis, hypoxia, cardiogenic shock accompanied by pulmonary edema.
Treatment. The most important therapeutic measures are to remove the drug from the body and restore stable functioning of the cardiovascular system.
After oral administration, complete gastric emptying is recommended, if necessary in combination with small intestinal lavage to prevent absorption of the active substance.
When using laxatives, it should be borne in mind that calcium antagonists lead to a decrease in intestinal muscle tone up to intestinal atony. Since nifedipine is characterized by a high degree of binding to blood plasma proteins and a relatively small volume of distribution, hemodialysis is ineffective, but plasmapheresis is recommended.
Bradycardia can be treated with β-sympathomimetics. In life-threatening bradycardia, the use of an artificial pacemaker is recommended.
Arterial hypotension resulting from cardiogenic shock and vasodilation can be treated with calcium preparations (10-20 ml of a 10% solution of calcium chloride or gluconate administered intravenously slowly, then repeated as needed). As a result, plasma calcium levels may reach the upper limit of normal or be slightly elevated. If calcium administration is not effective enough, it is advisable to use dopamine, dobutamine, epinephrine or norepinephrine. The doses of these drugs should be determined taking into account the achieved therapeutic effect. Additional fluid administration should be approached with great caution, since this increases the risk of cardiac overload.
Adverse reactions
From the side of the hematopoietic and lymphatic systems: changes in blood counts, anemia, leukopenia, thrombocytopenia and thrombotic microangiopathy, agranulocytosis.
Immune system disorders: allergic reactions, allergic edema/angioedema (including laryngeal edema, which may be life-threatening), pruritus, urticaria, rash, anaphylactic/anaphylactoid reaction, facial edema.
On the part of the psyche: anxiety, sleep disorders, mood swings, nervousness.
Metabolism: hyperglycemia.
Nervous system: headache, especially at the beginning of treatment; dizziness, migraine, tremor, paresthesia, dysesthesia, hypoesthesia, drowsiness, weakness, vertigo.
On the part of the organs of vision: slight temporary change in visual perception, visual impairment, eye pain.
Cardiovascular system: hot flashes, palpitations, tachycardia, edema (including peripheral edema), vasodilation, loss of consciousness, hypotension, myocardial infarction, chest pain (angina), erythromelalgia, especially at the beginning of treatment; thrombocytopenic purpura. At the beginning of therapy in patients with angina pectoris, an increase in the frequency, duration of attacks or an increase in the severity of symptoms is possible.
Respiratory system: epistaxis; nasal congestion; pulmonary edema (when used in pregnant women as a tocolytic agent); bronchial muscle spasm, including life-threatening dyspnea, which resolves after discontinuation of treatment.
On the part of the hepatobiliary system: transient increase in liver transaminase activity, jaundice, intrahepatic cholestasis.
Skin and subcutaneous tissue disorders: erythema, Mitchell's disease, especially at the beginning of treatment; hypersensitivity skin reactions such as itching, exanthema, swelling of the skin and mucous membranes, sweating, urticaria, photodermatitis, palpable purpura, exfoliative dermatitis, toxic epidermal necrolysis, photosensitivity reaction. In cases of long-term use of nifedipine, gingival hyperplasia is possible, which completely disappears after discontinuation of the drug.
Musculoskeletal system: myalgia, arthralgia, muscle cramps, joint swelling.
On the part of the kidneys and urinary tract: temporary decrease in renal function in patients with renal failure; pollakiuria, polyuria, dysuria, nocturia, increased frequency of urination, increased daily urine output.
From the reproductive system and mammary glands: gynecomastia (the process is reversible, the symptoms disappear after stopping nifedipine), erectile dysfunction.
General disorders: increased fatigue, feeling unwell, malaise, apathy, fever, nonspecific pain, chills, cold sweat.
Expiration date
3 years.
Storage conditions
The medicine does not require any special storage conditions. Keep out of the reach of children.
Packaging
10 tablets in a blister; 10 blisters in a box.
Vacation category
According to the recipe.
Producer
Merkle GmbH.
Location of the manufacturer and its business address
Ludwig-Merkle-Strasse 3, 89143 Blaubeuren, Germany.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.