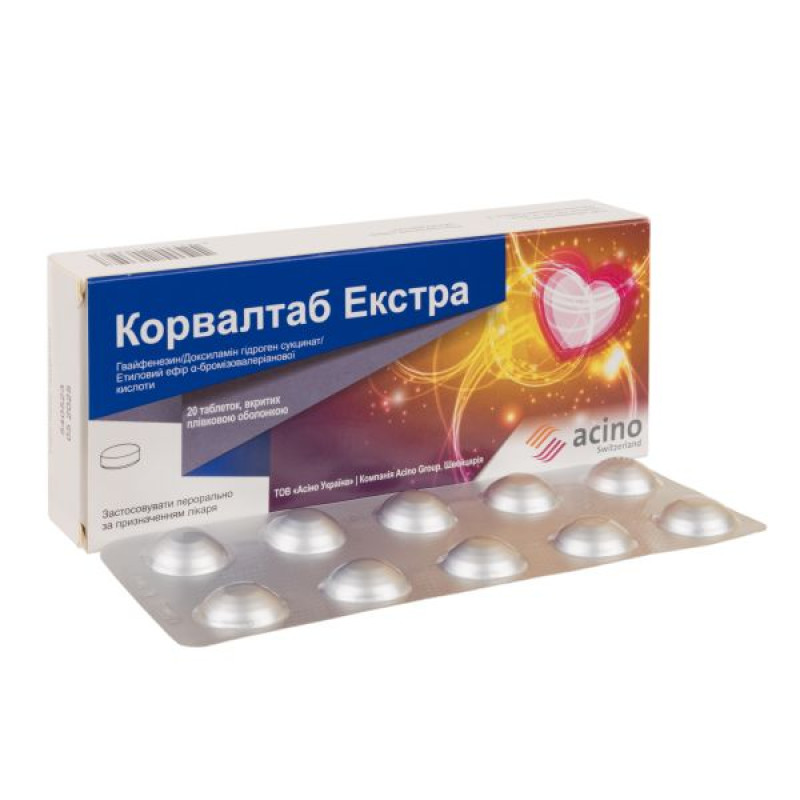
Corvaltab Extra film-coated tablets blister No. 20
Instructions for Corvaltab Extra film-coated tablets, blister pack No. 20
Composition
active ingredients: guaifenesin, doxylamine hydrogen succinate, α-bromoisovaleric acid ethyl ester;
1 tablet contains guaifenesin 100 mg, doxylamine hydrogen succinate 3.5 mg, α-bromoisovaleric acid ethyl ester 8.2 mg;
excipients: peppermint oil, β-cyclodextrin, maltodextrin, copovidone, crospovidone, silicon dioxide (colloidal hydrophobic), silicon dioxide colloidal aqueous, magnesium stearate; Opadry II White film coating mixture: polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide (E171).
Dosage form
Film-coated tablets.
Main physicochemical properties: round tablets with a biconvex surface, coated with a white film coating, with a specific odor.
Pharmacotherapeutic group
Hypnotics and sedatives. ATX code N05C M.
Pharmacological properties
Pharmacodynamics. Vasodilator (coronarolytic), sedative, hypnotic. Has a mild antianginal effect. Helps reduce the excitability of the central nervous system, has a calming effect and facilitates the onset of natural sleep.
The ethyl ester of α-bromosovaleric acid, which is part of the drug, has antispasmodic, coronarolytic and sedative effects; in large doses it also causes a mild hypnotic effect.
Guaifenesin exhibits anxiolytic (anti-anxiety) effects.
Doxylamine hydrogen succinate is a blocker of H1-histamine receptors and exhibits sedative, hypnogenic, and antiallergic effects.
Pharmacokinetics. Guaifenesin is rapidly (after 30 min) absorbed from the gastrointestinal tract. It mainly penetrates into tissues containing acidic mucopolysaccharides. After oral administration, the maximum concentration is reached after 1-2 h, and the therapeutic concentration is maintained for 6 h. The half-life of guaifenesin is about 1 h. It is excreted with sputum and excreted by the kidneys in the form of metabolites, as well as in an unchanged state. The maximum concentration of doxylamine hydrogen succinate in blood plasma (Cmax) is reached on average after 2 h (Tmax) after taking the drug.
The mean plasma half-life of doxylamine (T½) is on average 10 hours.
Doxylamine hydrogen succinate is partially metabolized in the liver by demethylation and N-acetylation. The elimination half-life may be significantly increased in the elderly and in patients with renal or hepatic insufficiency. The various metabolites formed during the breakdown of the molecule are not quantitatively significant, since 60% of the administered dose is found in the urine as unchanged doxylamine.
Indication
Mild coronary spasms; neurocirculatory dystonia - in complex therapy; neuroses with increased irritability; increased excitability; mild insomnia; dermatoses accompanied by itching.
Contraindication
Hypersensitivity to the components of the drug or to antihistamines. Severe renal and hepatic dysfunction, hepatic porphyria.
Angle-closure glaucoma in the patient's history or in the family history.
Uretroprostatic disorders with risk of urinary retention.
Severe heart failure.
Depression and other disorders accompanied by suppression of the central nervous system.
Special safety measures.
While taking the drug, you should avoid drinking alcoholic beverages.
It should be used with caution in elderly patients due to the risk of dizziness.
During treatment with the drug, sleep apnea syndrome may worsen (an increase in the number and duration of respiratory arrests).
In some cases, a change in urine color may occur.
The drug should be used with caution in the following categories of patients:
· with chronic or persistent cough caused by asthma, smoking, chronic bronchitis and emphysema;
with impaired renal function;
with myasthenia gravis;
with acute gastrointestinal disorders.
Interaction with other medicinal products and other types of interactions
When used simultaneously with other drugs that depress the central nervous system, mutual enhancement of the effect is possible, as well as enhancement of the effect of ethanol. The effect of the drug is enhanced against the background of alcohol intake.
It should be taken into account that when using a combination of the drug with:
- with atropine and atropine-like drugs (imipramine antidepressants, anticholinergic antiparkinsonian drugs, atropine antispasmodic drugs, disopyramide, phenothiazine neuroleptics) side effects such as urinary retention, constipation, dry mouth may occur;
- antidepressants, morphine derivatives (painkillers and cough suppressants), neuroleptics; barbiturates, benzodiazepines, centrally acting antihypertensives may increase central nervous system depression.
Use during pregnancy or breastfeeding
The drug is not prescribed to pregnant women and women during breastfeeding.
Ability to influence reaction speed when driving vehicles or other mechanisms
During the treatment period, you should refrain from driving or working with other mechanisms that require speed of psychomotor reactions.
Method of administration and doses
The dosage and duration of treatment are determined by the doctor individually for each patient. Adults are usually prescribed 1 tablet of the drug 2-3 times a day before meals. For mild insomnia, 1-2 tablets are prescribed 30 minutes before bedtime.
Children
There is no experience in the treatment of children, therefore the drug is not used in pediatric practice.
Overdose
Drowsiness, weakness, dizziness, vertigo, gastrointestinal disturbances and signs of anticholinergic effects may occur: agitation, dilated pupils, accommodation paralysis, dry mouth, flushing of the face and neck, hyperthermia, sinus tachycardia. Very high doses may cause symptoms such as agitation, confusion and respiratory depression.
Treatment: discontinuation of the drug, gastric lavage and symptomatic therapy. There is no specific antidote.
Adverse reactions
In some cases, the following side effects may occur:
from the nervous system: drowsiness, mild dizziness;
from the digestive tract: nausea, vomiting;
from the immune system: allergic reactions (including skin rash, itching, urticaria).
These phenomena disappear when the dose is reduced or the drug is discontinued.
With long-term use of large doses of the drug, the development of bromism is possible.
Diarrhea, constipation, dry mouth, accommodation disorders, palpitations.
Daytime drowsiness: if this effect develops, the dose should be reduced.
There have been rare reports of bladder or kidney stones in patients who have taken large amounts of guaifenesin over a long period of time.
In case of undesirable side reactions, you should consult a doctor.
Expiration date
2 years.
Storage conditions
Store out of the reach of children, in the original packaging at a temperature not exceeding 25 ºС.
Packaging
10 tablets in a blister; 1 or 2 blisters in a cardboard pack.
Vacation category
Without a prescription.
Manufacturer/Applicant
"Pharma Start" LLC.
Location of the manufacturer and its business address
Ukraine, Kyiv, I. Lepse Blvd., 8.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.