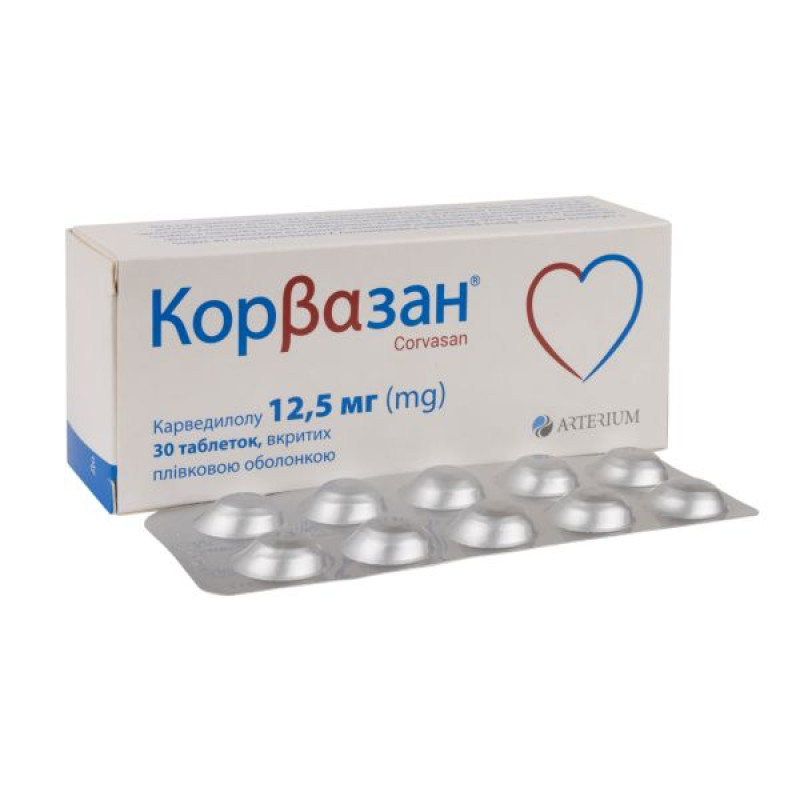
Corvazan film-coated tablets 12.5 mg No. 30

Instructions for use Corvazan film-coated tablets 12.5 mg No. 30
Composition
active ingredient: carvedilol;
1 tablet contains carvedilol in terms of 100% substance 12.5 mg or 25 mg;
excipients: microcrystalline cellulose; povidone; lactose monohydrate; potato starch; talc; calcium stearate; coating mixture "Opadry II Pink"*.
* The coating mixture "Opadry II Pink" contains triacetin; hypromellose; lactose monohydrate; titanium dioxide (E 171), polyethylene glycol, iron oxide yellow (E 172); iron oxide black (E 172); erythrosine (E 127); indigo carmine (E 132).
Dosage form
Film-coated tablets.
Main physicochemical properties: round tablets, film-coated in pink, with a biconvex surface, with two perpendicularly intersecting lines on one side of the tablet (for a dosage of 12.5 mg) or round tablets, film-coated in pink, with a biconvex surface, with a line and the inscription "KMP" on both sides of the line on one side of the tablet (for a dosage of 25 mg).
Pharmacotherapeutic group
Alpha- and beta-adrenergic blockers. Carvedilol.
ATX code C07A G02.
Pharmacological properties
Pharmacodynamics.
Carvedilol is a vasodilator, non-selective beta-blocker with antioxidant properties. The vasodilator effect is mainly due to selective blockade of alpha-1-adrenoreceptors. Carvedilol reduces peripheral vascular resistance by vasodilation and inhibition of the renin-angiotensin-aldosterone system activity as a result of blockade of beta-adrenoreceptors. Plasma renin activity is reduced, so fluid retention in the body is observed only in isolated cases.
Carvedilol has no sympathomimetic activity of its own and, like propranolol, exhibits membrane-stabilizing properties.
Carvedilol is a racemic mixture of 2 stereoisomers. Both stereoisomers have been shown to have alpha-adrenergic blocking properties in laboratory animal models. The beta-adrenergic blocking effects are not selective for beta-1 and beta-2 adrenoceptors and are associated with the levorotatory stereoisomer of carvedilol.
Carvedilol acts as a powerful antioxidant, reducing the number of active oxygen radicals.
Clinical data indicate that the vasodilator and beta-adrenergic blocking properties of carvedilol lead to the development of the following effects.
In patients with arterial hypertension, the decrease in blood pressure is not accompanied by a simultaneous increase in total peripheral vascular resistance, which is observed when taking true beta-blockers. Heart rate decreases slightly. Renal perfusion and renal function remain unchanged. Since peripheral blood circulation is also not changed, a feeling of coldness in the extremities (which is often observed when using true beta-blockers) is rarely observed.
In patients with coronary heart disease, carvedilol exhibits anti-ischemic and antianginal effects that are maintained with long-term use of the drug. Carvedilol reduces pre- and afterload on the heart.
In patients with impaired left ventricular function or chronic heart failure, the drug has a beneficial effect on hemodynamic parameters and improves left ventricular ejection fraction.
During treatment with carvedilol, the ratio of high-density lipoproteins to low-density lipoproteins (HDL/LDL) remains normal. Electrolyte balance is not disturbed.
Pharmacokinetics.
In men, the absolute bioavailability of carvedilol is approximately 25%. Peak serum concentrations are reached approximately 1 hour after oral administration. The relationship between dose and serum concentration is linear. Food intake does not affect bioavailability and peak serum concentrations, but increases the time to peak plasma concentrations. Carvedilol is a highly lipophilic compound: plasma protein binding is approximately 98-99%. The volume of distribution is approximately 2 l/kg, but is greater in patients with cirrhosis. Animal data suggest enterohepatic circulation of the parent compound.
Carvedilol is extensively metabolized to a number of metabolites, which are excreted mainly in the bile. Carvedilol is metabolized primarily in the liver, primarily by conjugation with glucuronic acid. Demethylation and hydroxylation of the phenolic ring lead to the formation of 3 active metabolites that have beta-adrenergic blocking activity. According to preclinical data, the 4-hydroxyphenolic metabolite is almost 13 times more active as a beta-adrenergic blocker than carvedilol. Compared with carvedilol, these three active metabolites have a weak vasodilator effect. In humans, their concentrations are 10 times lower than those of the parent compound. In addition, the two hydroxycarbazole metabolites of carvedilol are exceptionally potent antioxidants, with antioxidant activity 30-80 times greater than that of carvedilol.
Age does not affect the pharmacokinetics of the drug: in elderly patients, plasma levels of carvedilol are approximately 50% higher compared to those in young patients. There is evidence that the bioavailability of carvedilol in patients with liver cirrhosis is 4 times higher, and the maximum plasma concentration is 5 times higher than in healthy individuals.
There is evidence that in patients with arterial hypertension who have moderate (creatinine clearance 20-30 ml/min) or severe renal insufficiency (creatinine clearance < 20 ml/min), an increase in plasma carvedilol concentration by 40-55% is observed compared with that in patients with arterial hypertension whose renal function is within normal limits.
Indication
– Essential hypertension
– Chronic stable angina
– Chronic heart failure (adjunctive treatment).
Contraindication
- Hypersensitivity to the active substance or to any of the excipients of the drug;
- decompensated heart failure;
- NYHA class IV heart failure requiring intravenous inotropic agents;
- atrioventricular block of the II and III degree (except in cases where a permanent pacemaker is installed);
- concomitant intravenous administration of verapamil, diltiazem or other antiarrhythmic drugs (especially class I antiarrhythmic drugs);
- pronounced bradycardia (heart rate <50 beats/min);
- severe arterial hypotension (systolic pressure below 85 mm Hg);
- cardiogenic shock;
- sick sinus syndrome (including sinoatrial block);
- decompensated heart failure requiring intravenous administration of positive inotropic agents and/or diuretics;
- pulmonary heart, pulmonary hypertension;
- bronchial asthma or obstructive airway diseases accompanied by bronchospasm;
- pheochromocytoma (unless adequately controlled with alpha-blockers);
- Prinzmetal's angina;
- pronounced liver dysfunction;
- concomitant use of MAO inhibitors (except MAO-B inhibitors);
- galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption;
- metabolic acidosis.
Interaction with other medicinal products and other types of interactions
Carvedilol should be used with caution simultaneously with the following drugs:
− with agents that reduce catecholamine levels. Patients taking drugs with β-blocking properties and drugs that may reduce catecholamine levels (e.g. reserpine, guanethidine, methyldopa, guanfacine and monoamine oxidase inhibitors (except MAO-B inhibitors)) should be closely monitored for hypotension and/or severe bradycardia;
- with clonidine, since carvedilol and clonidine may potentiate each other's ability to lower blood pressure and reduce heart rate (HR); when used together, the drugs should be discontinued gradually: first, carvedilol should be discontinued, and then, after a few days, clonidine can be gradually discontinued;
- with digoxin, as atrioventricular conduction slows down and the level of digoxin in blood plasma increases;
- with calcium channel blockers (verapamil and diltiazem) and antiarrhythmics (especially class I), as they can provoke severe arterial hypotension and heart failure. Intravenous administration of these drugs simultaneously with carvedilol is contraindicated;
- with insulin and oral antidiabetic agents (due to increased hypotensive effect and masking of symptoms of hypoglycemia);
- with nitrates and antihypertensive agents (clonidine, guanethidine, reserpine, alpha-methyldopa, guanfacine) due to increased hypotensive effect and decreased heart rate;
- with anesthetics (due to their negative inotropic effect and hypotensive effect);
- with drugs that affect the CNS (hypnotics, tranquilizers, tricyclic antidepressants and ethyl alcohol) - due to the possibility of mutual enhancement of effects;
- with nonsteroidal anti-inflammatory drugs (due to reduced hypotensive effect (due to reduced production of prostaglandins));
- with alpha- and beta-sympathomimetics (due to the possible development of arterial hypertension, pronounced reflex bradycardia and asystole, as well as a decrease in the beta-adrenergic blocking effect of carvedilol);
- with xanthine derivatives (aminophylline, theophylline) - due to a decrease in beta-adrenergic blocking effect.
Since carvedilol undergoes oxidative metabolism, its pharmacokinetics may change upon induction or inhibition of the cytochrome P450 enzyme system, therefore the effect of:
- rifampicin (there is a 70% decrease in the concentration of carvedilol in blood plasma);
- barbiturates (reduce the effectiveness of carvedilol);
- cimetidine (increases the bioavailability of carvedilol by 30%);
- digoxin (carvedilol increases the concentration of digoxin in blood plasma);
- CYP2D6 isoenzyme inhibitors (quinidine, paroxetine, propafenone): an increase in the concentration of the R(+) enantiomer of carvedilol can be assumed;
- carvedilol delays the metabolism of cyclosporine.
Application features
The drug should be used with extreme caution in the following conditions: unstable or secondary arterial hypertension in the absence of sufficient clinical experience, right bundle branch block, acute carditis, impaired function of heart valves and hemodynamic disorders, bradycardia, chronic heart failure, left ventricular failure on the background of acute myocardial infarction, Raynaud's phenomenon, thyrotoxicosis, pheochromocytoma, chronic obstructive pulmonary diseases, anesthesia, simultaneous administration with calcium channel blockers, clonidine.
Patients with severe heart failure (above NYHA class III), electrolyte imbalance, low blood pressure (less than 100 mm Hg) or elderly (over 70 years of age) should be under close medical supervision for 2 hours after taking the first dose or after taking the first increased dose due to the risk of developing a sudden decrease in blood pressure, postural hypotension and loss of consciousness. The risk of these complications can be reduced by prescribing the drug in small initial doses and taking it with food.
In case of bradycardia (heart rate less than 55 beats/min), the dose of the drug should be reduced.
If carvedilol is used to treat hypertension or angina in patients with heart failure, therapy with the drug should be carried out according to the regimen recommended for the treatment of heart failure: the initial dose should not exceed 3.125 mg twice daily, and any dose increase should be carried out after at least 2 weeks of treatment with the previous dose of the drug.
The use of carvedilol in patients with hypertension who are already receiving digitalis, diuretics and/or ACE inhibitors requires close medical supervision, as all of these drugs may reduce atrioventricular conduction.
In patients with chronic heart failure, if the systolic blood pressure is less than 100 mm Hg or there are concomitant diseases - ischemic heart disease, peripheral vascular disease or impaired renal function - renal function should be checked more often. If renal function is impaired, the dose of the drug should be reduced or treatment discontinued.
In patients with Prinzmetal's angina, non-selective beta-blockers may cause chest pain (the alpha1-adrenergic blocking effect of carvedilol may prevent this, but there is insufficient clinical experience with carvedilol in Prinzmetal's angina).
The use of the drug for unstable angina, as well as for first-degree atrioventricular block, requires special caution, frequent ECG recording, and close medical supervision of patients.
Treatment of patients with peripheral arterial disease requires attention and caution.
Like other beta-blockers, carvedilol may mask the symptoms of hypoglycemia and adversely affect carbohydrate metabolism. Therefore, treatment with carvedilol in patients with diabetes requires special attention and more frequent measurement of blood sugar levels.
Carvedilol may mask the symptoms of hyperthyroidism. Abrupt withdrawal of the drug may lead to an increase in thyrotoxicosis and the development of a thyrotoxic crisis.
Treatment should be discontinued by gradually reducing the dose, especially in chronic stable angina. If necessary, other antianginal agents can be used during the withdrawal period.
Carvedilol treatment of patients with established pheochromocytoma should not be initiated until adequate therapeutic alpha-adrenergic blockade has been established.
Given the risk of developing extreme anaphylactic reactions, the drug should be used with extreme caution in patients with a history of hypersensitivity reactions or who have previously undergone hyposensitizing therapy.
Carvedilol treatment of patients with psoriasis requires careful assessment of the risk/benefit ratio, as carvedilol may exacerbate or trigger psoriasis symptoms.
Each tablet of the drug contains lactose, so it should not be used in case of lactase deficiency, galactosemia and glucose/galactose malabsorption syndrome.
Abrupt discontinuation of carvedilol (as with other beta-blockers) may cause sweating, tachycardia, shortness of breath, worsening of angina pectoris, and even myocardial infarction and ventricular arrhythmias. Patients with angina pectoris who are at risk of a heart attack are at greatest risk. The dose should be reduced gradually over 1-2 weeks. If necessary, replacement therapy can be started at the same time to prevent exacerbation of the disease. If treatment has been temporarily discontinued for more than 2 weeks, it should be resumed starting with the lowest dose.
In patients with chronic heart failure, when the dose of carvedilol is increased for the purpose of its selection, an increase in heart failure symptoms and the appearance of edema are possible.
If such symptoms occur, the dose of the diuretic should be adjusted, and the dose of carvedilol should not be increased until the clinical condition has stabilized. Sometimes it may be necessary to reduce the dose of carvedilol or temporarily discontinue its use.
Carvedilol should be used with particular caution in patients undergoing surgery under general anesthesia when using anesthetics such as ether, cyclopropane, trichloroethylene, which suppress myocardial function.
Use during pregnancy or breastfeeding
Clinical experience with carvedilol during pregnancy is limited.
It is assumed that the use of carvedilol in the fetus or newborn may cause distress syndrome (bradycardia, hypotension, respiratory depression, hypoglycemia and hypothermia). Therefore, the use of carvedilol during pregnancy is contraindicated.
It is not known whether carvedilol passes into breast milk. Since carvedilol may have harmful effects on the infant, treatment with carvedilol should be discontinued during breast-feeding.
Ability to influence the reaction speed when driving vehicles or other mechanismsb
At the beginning of treatment with carvedilol, dizziness and increased fatigue may occur, so they should refrain from driving vehicles and working with potentially dangerous mechanisms.
Method of administration and doses
The tablets should be taken with meals and with sufficient liquid.
Essential hypertension.
The recommended frequency of taking the drug is once a day.
The recommended initial dose is 12.5 mg once daily for the first 2 days of treatment. The recommended dose is then 25 mg daily. If necessary, the dose may be gradually increased at intervals of at least two weeks to a maximum recommended dose of 50 mg daily, which should be taken as a single dose or in two divided doses.
The drug can be used both as monotherapy and in combination with other antihypertensive drugs, especially thiazide diuretics.
Elderly patients
The recommended starting dose is 12.5 mg once daily. If the effect is insufficient, the dose can be gradually increased at intervals of at least two weeks to a maximum recommended daily dose of 50 mg.
Chronic stable angina.
The recommended starting dose is 12.5 mg twice daily for the first 2 days of treatment. The recommended dose is then 25 mg twice daily. If necessary, the dose may be increased at intervals of at least two weeks to a maximum recommended daily dose of 100 mg, which should be given in two divided doses.
Elderly patients
The recommended initial dose is 12.5 mg 2 times a day for 2 days.
Then continue treatment at a dose of 25 mg 2 times a day, which is the recommended maximum daily dose.
Chronic heart failure.
The dose of the drug should be set individually, and its gradual increase should be carried out under the close supervision of a doctor.
Carvedilol can be used as an adjunct to standard therapy, but it can also be used in patients who are intolerant to ACE inhibitors or those patients receiving digitalis, hydralazine, or nitrates.
Patients taking digoxin, diuretics or ACE inhibitors should receive these drugs at a pre-established dose before starting treatment with carvedilol.
The recommended starting dose is 3.125 mg twice daily for 2 weeks. If this dose is well tolerated, it may be increased gradually at intervals of at least 2 weeks, initially to 6.25 mg twice daily, then to 12.5 mg twice daily, and finally to 25 mg twice daily. The dose should be increased to the highest dose tolerated by the patient.
At the beginning of therapy or when increasing the dose, a temporary increase in heart failure symptoms may occur, especially in patients with severe heart failure and/or in those patients who are taking high doses of diuretics. Although in this case, discontinuation of treatment is usually not necessary, the dose of the drug should not be increased. During the first 2 hours after starting treatment with carvedilol or increasing its dose, the patient should be under the supervision of a cardiologist or other physician. Before each increase in the dose of the drug, the patient should be examined for possible symptoms of worsening heart failure or symptoms of excessive vasodilation (e.g., renal function, weight, blood pressure, heart rate and heart rhythm). Symptoms of worsening heart failure or fluid retention in the body are eliminated by increasing the dose of diuretics, but the dose of carvedilol should not be increased until the patient's condition is stabilized. If bradycardia occurs or in the event of prolonged atrioventricular conduction, the plasma digoxin level should be checked first. In some cases, it may be necessary to reduce the carvedilol dose or temporarily discontinue the drug altogether. Even in such cases, carvedilol can be successfully continued to be titrated.
During dose titration, renal function, platelet count and blood glucose levels (in non-insulin-dependent diabetes mellitus and/or insulin-dependent diabetes mellitus) should be monitored regularly. However, once dose titration is complete, the frequency of monitoring may be reduced.
If carvedilol treatment has been interrupted for more than 2 weeks, it should be resumed, starting with a dose of 3.125 mg twice daily and gradually increasing it according to the recommendations above.
Elderly patients
Elderly patients may be more sensitive to the effects of carvedilol and should therefore be monitored more closely.
Patients with hepatic insufficiency
Carvedilol should not be administered to patients with severe hepatic impairment (see section 4.3). Dose adjustment may be required in patients with moderate hepatic impairment.
Patients with renal insufficiency
The dose should be individualized for each patient, but there are no data to suggest that carvedilol dosage adjustments are necessary for patients with renal insufficiency.
Discontinuation of treatment.
Carvedilol treatment should not be stopped abruptly, especially in patients with coronary artery disease. Discontinuation should be gradual over 7-10 days, for example by halving the daily dose every three days.
Children
The safety and efficacy of the drug in children have not been established, therefore the drug is not intended for use in this category of patients.
Overdose
In case of overdose, severe hypotension, bradycardia, heart failure, cardiogenic shock, cardiac arrest may develop. Difficulty breathing, bronchospasm, vomiting, impaired consciousness and generalized convulsions are also possible.
Treatment: during the first hours - induce vomiting and wash the stomach, then - monitor and correct vital signs in the intensive care unit.
Supportive therapy
Atropine: 0.5-2 mg intravenously (for treatment of severe bradycardia).
Glucagon: initially at a dose of 1-10 mg intravenously, and then as a drip infusion at a rate of 2-5 mg/h (to maintain cardiovascular function).
Sympathomimetics – dobutamine, isoprenaline, orciprenaline or adrenaline – should be prescribed depending on the patient's body weight and the effectiveness of the treatment.
If the clinical picture of overdose is dominated by peripheral vasodilation, then adrenaline or noradrenaline should be administered under constant monitoring of the patient's cardiovascular system. In the case of bradycardia resistant to drug treatment, the use of an artificial pacemaker is indicated. In the event of bronchospasm, the administration of beta-sympathomimetics (in the form of an aerosol or intravenously) or intravenous administration of aminophylline is indicated. In the case of generalized convulsions, slow intravenous administration of diazepam or clonazepam is recommended.
Caution: In case of severe overdose accompanied by symptoms of shock, supportive therapy with antidotes should be continued for a sufficiently long time, since an increase in the half-life of carvedilol and its redistribution can be expected. The duration of treatment depends on the degree of overdose; supportive therapy should be continued until the patient's condition stabilizes.
Adverse reactions
From the side of the central nervous system: headache, dizziness, increased fatigue; depression, sleep disturbances, paresthesia, hypoesthesia, vertigo, state before loss of consciousness, loss of consciousness.
From the psyche: depressive mood.
Vascular disorders: arterial hypotension, peripheral vascular disease.
Respiratory system: shortness of breath, bronchial asthma, bronchospasm; nasal congestion.
Respiratory, thoracic and mediastinal disorders: pulmonary edema, bronchial asthma in susceptible patients, rhinitis.
On the part of the digestive tract: nausea, diarrhea, abdominal pain; dry mouth, constipation, vomiting, increased intestinal tone and motility, periodontitis, melena, dyspepsia.
Liver and biliary tract disorders: increased alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gammaglutamyltransferase (GGT).
Skin and subcutaneous tissue disorders: skin reactions (including: allergic exanthema, dermatitis, increased sweating, urticaria, itching; skin lesions similar to psoriasis and lichen planus), psoriatic skin lesions or worsening of a pre-existing condition, alopecia.
On the part of the organs of vision: decreased lacrimation, visual impairment, eye irritation.
Metabolism and digestion: hypoglycemia, impaired blood glucose regulation, anorexia/weight loss, weight gain.
Musculoskeletal system: joint pain, arthralgia, cramps, muscle atrophy.
From the reproductive system and mammary glands: erectile dysfunction.
Renal and urinary disorders: acute renal failure and renal dysfunction in patients with diffuse vascular disease and/or renal failure, urinary incontinence in women, hematuria, albuminuria.
Immune system disorders: hypersensitivity reactions; angioedema; Stevens-Johnson syndrome, toxic epidermal necrolysis, erythema multiforme.
General disorders: asthenia (including fatigue), pain.
Laboratory indicators: increased serum transaminase levels, thrombocytopenia, leukopenia, anemia, decreased prothrombin levels, hyperglycemia in patients with diabetes mellitus, hypercholesterolemia, glucosuria, hyperkalemia, hypertriglyceridemia, hyponatremia, increased alkaline phosphatase, creatinine, urea, hyperuricemia.
Other side effects: flu-like symptoms, fever; infections; bronchitis, pneumonia, upper respiratory tract infection, urinary tract infection; anaphylactic reactions, possible manifestations of latent diabetes mellitus, symptoms of existing diabetes mellitus may worsen during therapy; flushing. With the exception of dizziness, visual disturbances and bradycardia, none of the side effects described above are dose-dependent.
Expiration date
2 years.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 ºС, out of the reach of children.
Packaging
10 tablets in a blister, 3 blisters in a pack.
Vacation category
According to the recipe.
Producer
PJSC "Kyivmedpreparat".
Location of the manufacturer and its address of the place of implementation of the activity
01032, Ukraine, Kyiv, Saksaganskoho St., 139.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.