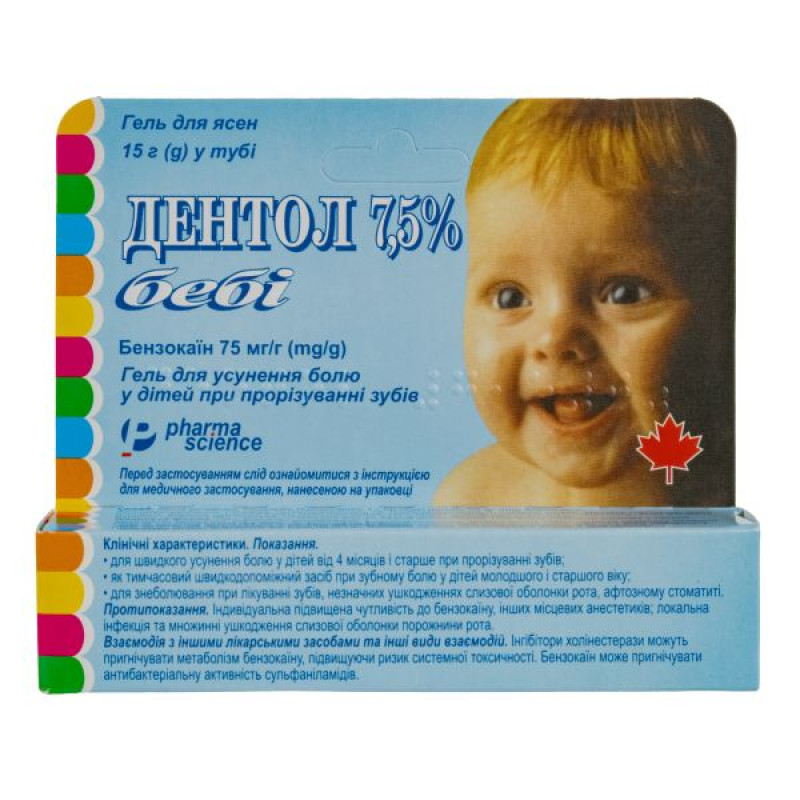
Dentol 7.5% gel for gums 75 mg/g tube of 15 g
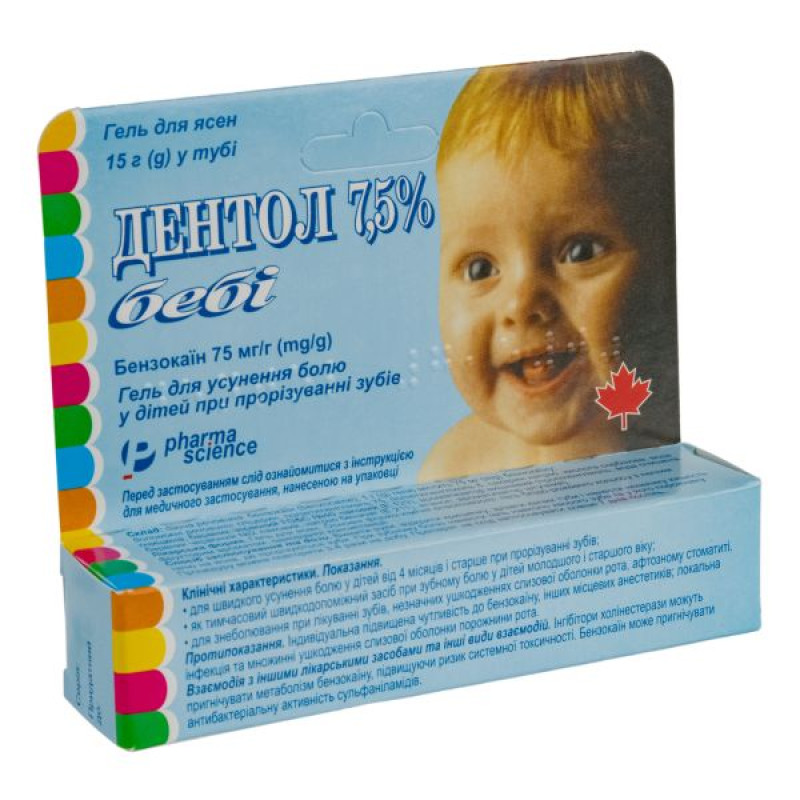
Instructions Dentol 7.5% gum gel 75 mg/g tube 15 g
Composition
active ingredient: benzocaine;
Dentol 7.5%, 1 g of gel contains 75 mg of benzocaine;
excipients: glycerin, polyethylene glycol 75, polyethylene glycol 8, sodium saccharin, sorbic acid, purified water, FD&C red dye No. 40 lake (E 129), cherry flavoring (propylene glycol, purified water, red dye No. 2 lake, sodium benzoate (E 211), ethanol, artificial flavoring).
Dentol 10%, 1 g of gel contains 100 mg of benzocaine;
excipients: glycerin, polyethylene glycol 75, polyethylene glycol 8, sodium saccharin, sorbic acid, purified water, cherry flavoring (propylene glycol, purified water, red dye No. 2 lake, sodium benzoate (E 211), ethanol, artificial flavoring).
Dosage form
Gum gel.
Main physicochemical properties: pale pink gel with a light fruity aroma, homogeneous consistency.
Pharmacotherapeutic group
Local anesthesia agents.
ATX code N01B A05.
Pharmacological properties
Pharmacodynamics.
Benzocaine is a local anesthetic that reduces the permeability of membranes to sodium ions and blocks the conduction of nerve impulses.
Pharmacokinetics.
Benzocaine is poorly adsorbed, the action begins 1 minute after application and lasts 15-20 minutes. The adsorbed drug is rapidly hydrolyzed by blood and liver cholinesterases, and excreted by the kidneys.
Indication
Dentol 7.5%:
for quick relief of pain in children aged 4 months and older during teething;
as a temporary first aid remedy for toothache in young and older children;
for pain relief during dental treatment, minor injuries to the oral mucosa, and aphthous stomatitis.
Dentol 10%:
for quick relief of toothache in adults and children aged 6 and over;
as a temporary first aid remedy, before consulting a doctor, for sore gums, during dental treatment and prosthetics, and for minor injuries to the oral mucosa;
as an anesthetic agent when providing dental care.
Contraindication
Individual hypersensitivity to benzocaine, other local anesthetics; local infection and multiple lesions of the oral mucosa.
Interaction with other medicinal products and other types of interactions
Cholinesterase inhibitors may inhibit the metabolism of benzocaine, increasing the risk of systemic toxicity. Benzocaine may inhibit the antibacterial activity of sulfonamides.
Application features
Do not exceed the dose, frequency, and duration of administration. It is not recommended to use the drug more than 4 times a day and for longer than 7 consecutive days.
If symptoms do not disappear within 7 days or irritation, pain, redness do not disappear or worsen, or swelling, rash, fever appear, you should immediately consult a doctor.
There have been reports that the use of products containing benzocaine may cause methemoglobinemia. Symptoms such as cyanosis of the skin, lips, and nail beds, headache, dizziness, dyspnea (difficulty breathing), weakness, and tachycardia that may occur during treatment may indicate methemoglobinemia, which is potentially life-threatening and requires immediate medical attention.
Use during pregnancy or breastfeeding
Use with caution during pregnancy or breastfeeding, taking into account the benefit-risk ratio.
Ability to influence reaction speed when driving vehicles or other mechanisms
The drug does not affect driving or working with other mechanisms.
Method of administration and doses
Wash your hands, open the cap, cut off the tip of the tube and apply a small amount of gel to the gums where the teeth are erupting. For toothache, apply the gel around the affected tooth and into its cavity. The procedure can be repeated 3-4 times a day.
The drug should not be used for longer than 7 consecutive days.
Children
Do not use the drug in children under 4 months of age.
It is recommended to use the drug for the treatment of children under 2 years of age under the supervision of a doctor or medical professional.
Overdose
Overdose is unlikely if all recommendations are followed. In some cases, toxic effects on the central nervous system are possible (visual disturbances, convulsions, tinnitus, drowsiness, feeling of heat or cold, agitation, confusion, dizziness, feeling of numbness, anxiety, restlessness); depression of the cardiovascular system (sweating, arterial hypotension, pallor, arrhythmia, cardiac arrest); development of methemoglobinemia.
Treatment. In case of overdose of the drug, rinse your mouth with a warm solution of soda and urgently consult a doctor. Therapy is symptomatic.
Side effects
Side effects may occur due to exceeding the recommended doses, increased absorption rate, hypersensitivity, including allergic contact dermatitis (skin rash, redness, itching or hives); angioedema (swelling of the skin or mucous membranes of the mouth and throat); burning sensation, fever. If these symptoms appear, you should stop using the drug and consult a doctor.
When using benzocaine, methemoglobinemia (cyanosis of the skin, lips and nail beds, headache, dizziness, shortness of breath, weakness, tachycardia) is very rarely possible.
Expiration date
4 years.
Storage conditions
Store out of the reach of children at a temperature not exceeding 30 °C.
Packaging
15 g in a tube, 1 tube in a cardboard box.
Vacation category
Without a prescription.
Producer
Pharmascience Inc.
Address
6111 Royalmount Avenue, 100, Montreal, Quebec H4P 2T4, Canada/6111 Royalmount Avenue, 100, Montreal, Quebec H4P 2T4, Canada.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.