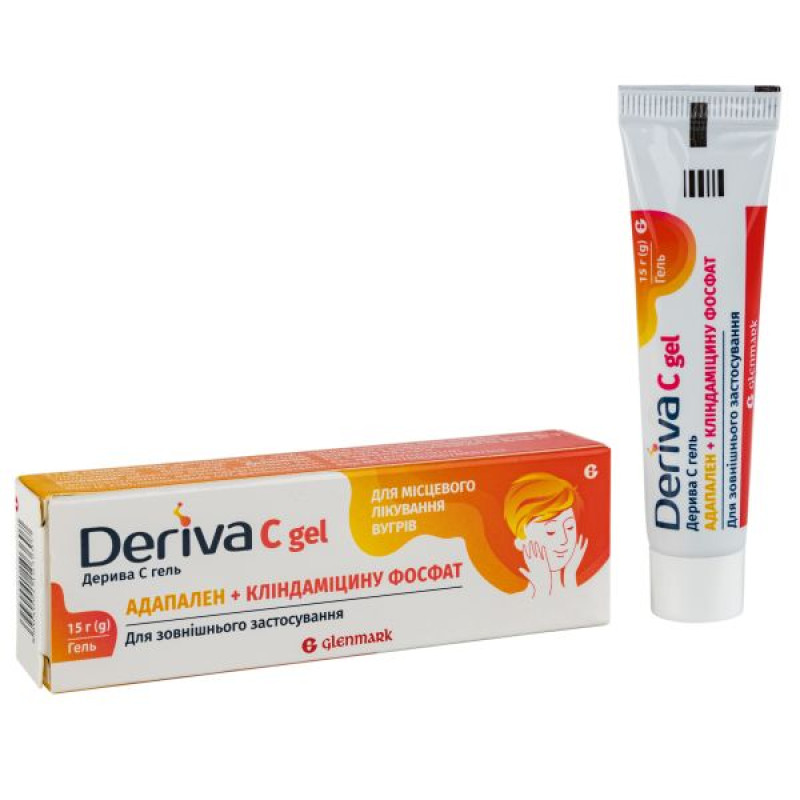
Deriva C gel tube 15 g
Instructions for Deriva C gel tube 15 g
Composition
active ingredients: adapalene, clindamycin;
1 g of gel contains adapalene 1 mg, clindamycin (as clindamycin phosphate) 10 mg;
Excipients: disodium edetate, carbomer 940, propylene glycol, methyl parahydroxybenzoate (E 218), poloxamer 407, phenoxyethanol, sodium hydroxide, purified water.
Dosage form
Gel.
Main physicochemical properties: homogeneous gel of white or pale yellow color.
Pharmacotherapeutic group
Acne treatment drugs for topical use.
ATX code D10A D53.
Pharmacological properties
Pharmacodynamics. Adapalene is a derivative of naphthoic acid, a retinoid-like substance that modulates the processes of cellular differentiation and keratinization, as well as skin inflammation, which are the main pathogenetic links in the development of acne. Adapalene binds to retinoid receptors in the cell nucleus and, thus, promotes normal differentiation of follicular epithelial cells, which leads to a decrease in the formation of microcomedones and prevents the development of acne, helps to preserve intact skin.
The therapeutic effect of the drug usually appears within 8-12 weeks from the start of treatment. When using adapalene in the form of a gel, absorption of the drug through the skin into the blood is extremely low.
Clindamycin phosphate is a semisynthetic antibiotic that acts as an inhibitor of bacterial protein synthesis by binding to the 50S subunit of the ribosome and inhibiting the initiation of peptide chain formation. Clindamycin inhibits all cultures of Propionibacterium aspes tested with a minimum inhibitory concentration of 0.4 μg/ml. Cross-resistance between clindamycin and erythromycin has been demonstrated.
Pharmacokinetics: Not studied.
Indication
Topical treatment of common acne (acne vulgaris).
Contraindication
Hypersensitivity to adapalene, clindamycin or other components of the drug, as well as to lincomycin. Enteritis, ulcerative colitis, antibiotic-associated colitis (history), Crohn's disease.
Interaction with other medicinal products and other types of interactions
Since the drug may have a local irritant effect in some patients, the simultaneous use of other potentially irritating local drugs increases the risk of undesirable effects on the skin.
The gel should be used with caution with preparations containing sulfur, resorcinol, or salicylic acid.
If it is necessary to use the drug together with other medications, apply the gel once a day at night, and apply other medications in the morning.
Clindamycin has the property of blocking neuromuscular transmission, which may lead to an increase in the action of other drugs with similar properties. Therefore, it should be used with caution in patients receiving such drugs. There is cross-resistance between clindamycin and lincomycin. Antagonism between erythromycin and clindamycin has also been noted.
Application features
The drug is intended for topical use only.
Avoid contact of the gel with the eyes, lips, nasal wings and skin around the eyes, as well as with mucous membranes. If the gel accidentally gets on these areas, rinse them thoroughly with warm water.
Do not apply the gel to eczematous skin lesions, sunburns, cuts or other skin lesions.
During treatment, excessive exposure to sunlight and ultraviolet light, including lamps, should be avoided due to increased skin sensitivity and an increased risk of solar erythema.
Use during treatment with cosmetics that dry the skin (abrasive or medicated soaps, skin cleansers, products containing excessive amounts of alcohol, astringents, creams or lotions for or after shaving, detergents) may lead to an irritating effect.
In the event of an allergic reaction to any of the components of the drug, therapy should be discontinued and appropriate measures should be taken.
When clindamycin is applied topically, the antibiotic is absorbed from the skin surface. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been reported with topical and systemic use of clindamycin.
Studies show that the main cause of antibiotic-associated colitis is a toxin produced by Clostridium difficile. Colitis is usually characterized by severe, persistent diarrhea and severe abdominal cramps, and may be accompanied by blood and mucus. Pseudomembranous colitis can be detected by endoscopic examination. Stool culture for Clostridium difficile and stool analysis for Clostridium difficile toxin can help in the diagnosis of this disease.
The use of agents that depress peristalsis, such as opiates and diphenoxylate with atropine, may prolong and/or worsen this condition. Vancomycin has been shown to be effective in the treatment of antibiotic-associated pseudomembranous colitis caused by Clostridium difficile. The usual dose for adult patients is 500 mg to 2 g of vancomycin per day orally in 3 to 4 divided doses for 7 to 10 days. Cholestyramine or colestipol resins bind vancomycin in vitro. If concomitant use of the resin and vancomycin is necessary, it is advisable to use each of these drugs at different times.
Given the potential for diarrhea, bloody diarrhea, and pseudomembranous colitis, the physician should decide whether other medications should be used (see sections "Contraindications", "Adverse Reactions").
It is necessary to avoid contact of the drug with the mucous membrane of the eyes and the oral cavity. When applying the gel, wash your hands thoroughly. In case of accidental contact with sensitive surfaces (eyes, abrasions on the skin, mucous membranes), rinse the affected area thoroughly with cool water.
Oral and parenteral use of clindamycin has been associated with severe colitis, which can be fatal. Caution should be exercised when prescribing topical formulations of clindamycin phosphate to patients with atopy.
Use during pregnancy or breastfeeding
Since adequate clinical trials on the safety of the drug in pregnant women have not been conducted, the gel should not be used during this period.
The use of the drug during breastfeeding is contraindicated.
Ability to influence reaction speed when driving vehicles or other mechanisms
No effect.
Method of administration and doses
The drug should be used by adults and children over 12 years of age.
Apply the gel in a thin layer to clean, dry skin in the areas of rashes once a day, at night.
During the first weeks of treatment, the acne process may worsen due to the effect of the active substance on previously invisible lesions. In this case, treatment should not be discontinued, the therapeutic effect is observed 8-12 weeks after the start of treatment.
Children.
The safety and efficacy of the drug in children under 12 years of age have not been established, therefore the drug should not be used in children of this age group.
Overdose
Applying too much gel may cause redness and peeling of the skin.
Therapy is symptomatic.
Adverse reactions.
Redness, peeling, dryness, itching and burning of the skin at the site of application of the gel immediately after its application, which passes later. Allergic reactions, photosensitivity reactions, acne, tingling sensation, gram-negative folliculitis, gastrointestinal disorders, abdominal pain, urticaria, increased oiliness of the skin, contact dermatitis, burning of the eyes, rarely observed adverse reactions in the form of diarrhea, bloody diarrhea and colitis (including pseudomembranous colitis).
The following adverse reactions have been reported with oral or parenteral use of clindamycin.
Infections and infestations: Clostridial colitis (caused by Clostridium difficile).
Hypersensitivity reactions: maculopapular rash and urticaria. Generalized skin rashes of mild to moderate severity were most commonly reported. Cases of acute generalized exanthematous pustulosis, erythema multiforme, some resembling Stevens-Johnson syndrome, have been reported in association with clindamycin. A few cases of anaphylactoid reactions have been reported. If a hypersensitivity reaction occurs, the drug should be discontinued.
Musculoskeletal disorders: polyarthritis.
Renal and urinary disorders: acute kidney injury.
Immune system disorders: drug reaction with eosinophilia and systemic symptoms (DRESS syndrome).
Side effects
Redness, peeling, dryness, itching and burning of the skin at the site of application of the gel immediately after its application, which passes later. Allergic reactions, photosensitivity reactions, acne, tingling sensation, gram-negative folliculitis, gastrointestinal disorders, abdominal pain, urticaria, increased oiliness of the skin, contact dermatitis, burning of the eyes, rarely observed adverse reactions in the form of diarrhea, bloody diarrhea and colitis (including pseudomembranous colitis).
Expiration date
2 years.
Storage conditions
Store at a temperature not exceeding 25 ° C. Do not freeze. Keep out of the reach of children.
Packaging
15 g of gel in a tube, 1 tube in a cardboard box.
Vacation category
According to the recipe.
Producer
Glenmark Pharmaceuticals Ltd./Glenmark Pharmaceuticals Ltd.
Location of the manufacturer and its business address.
Precinct No. E-37/39, M.I.D.C., Satpur, Nasik – 422 007, India/
Plot No E-37/39, MIDC, Industrial Estate, Satpur, Nasik – 422 007, India.
Address
Precinct No. E-37/39, M.I.D.C., Satpur, Nasik – 422 007, India/
Plot No E-37/39, MIDC, Industrial Estate, Satpur, Nasik – 422 007, India.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.