Diane-35 film-coated tablets blister pack No. 21
Instructions Diane-35 film-coated tablets blister pack No. 21
Composition
active ingredients: ethinylestradiol, cyproterone acetate;
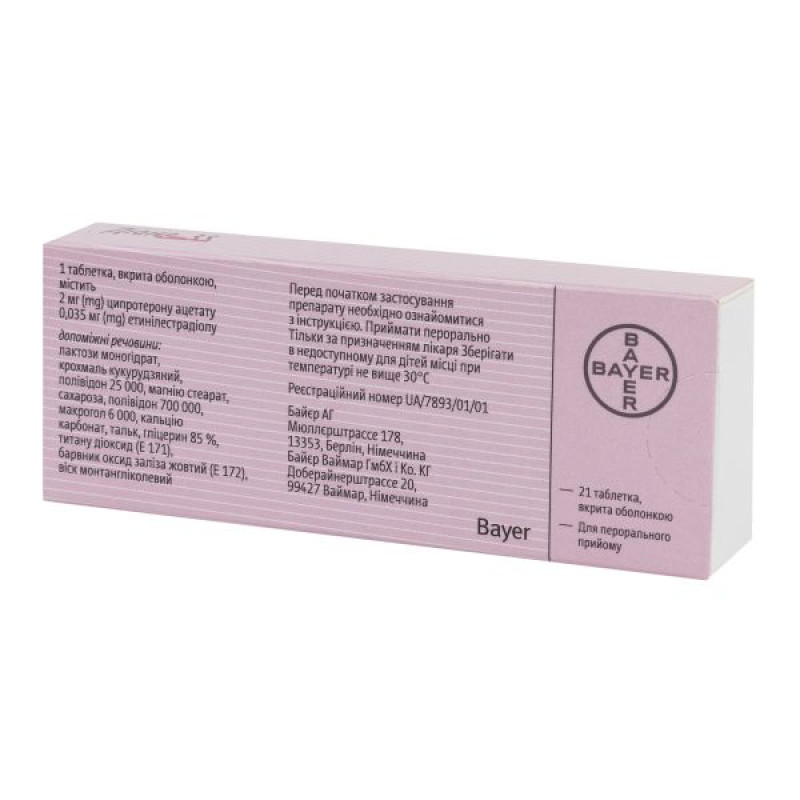
1 film-coated tablet contains ethinylestradiol 0.035 mg, cyproterone acetate 2 mg;
excipients: lactose monohydrate, corn starch, polyvidone 25000, magnesium stearate, sucrose, polyvidone 700000, macrogol 6000, calcium carbonate, talc, glycerin 85%, titanium dioxide (E 171), iron oxide yellow dye (E 172), montan glycol wax.
Dosage form
Film-coated tablets.
Main physicochemical properties: beige, film-coated, round, biconvex tablets.
Pharmacotherapeutic group
Sex hormone and drugs used in the pathology of the genital area. Antiandrogens and estrogens. ATX code G03HB.
Pharmacological properties
Pharmacodynamics
Hair follicles and sebaceous glands are sensitive to the action of androgens. The development of acne and seborrhea is due in particular to impaired function of the sebaceous glands, which develops as a result of increased peripheral sensitivity to androgens or increased levels of androgens in the blood plasma. Both active substances that make up Diane-35 have a positive therapeutic effect. Cyproterone acetate competitively replaces androgens in the effector organ and thus eliminates the androgenic effect. The concentration of androgens in the blood plasma is further reduced due to the antigonadotropic effect. This antigonadotropic effect is enhanced by ethinylestradiol, which also activates sex steroid binding globulin (SGB) in the blood plasma. In view of this, the level of unbound, bioavailable androgen in the blood plasma is reduced. When using Diane-35 (usually after 3-4 months of therapy), acne disappears. Excessive oiliness of the hair and skin usually disappears even earlier. Androgen-dependent hair loss also decreases.
When Diane-35 is used in women to treat hirsutism, the effect is slow to appear. Clinically significant results of therapy can be expected several months after its initiation.
Cyproterone acetate is also a potent progestogen that exerts its contraceptive effect when used in combination with ethinylestradiol. Its effect is due to the interaction of central and peripheral mechanisms, of which the suppression of ovulation and changes in cervical secretion are considered the most important. In addition, morphological and enzymatic changes occurring in the endometrium create extremely unfavorable conditions for implantation.
When using the drug according to the instructions, contraceptive protection is available from the first day of use.
Pharmacokinetics
Cyproterone acetate
Absorption. After oral administration, cyproterone acetate is completely absorbed over a wide range of doses. Its maximum serum concentration is 15 ng/ml and is reached approximately 1.6 hours after administration. The absolute bioavailability of cyproterone acetate is approximately 88%. The relative bioavailability of cyproterone acetate when administered with Diane-35 was 109% compared to an aqueous microcrystalline suspension.
Distribution. Cyproterone acetate in serum is almost entirely protein-bound. Only 3.5-4% of the total steroid concentration remains unbound, the rest is bound to albumin. Since the binding of cyproterone acetate to GSH is nonspecific, changes in GSH levels caused by ethinylestradiol do not affect the pharmacokinetics of cyproterone acetate.
Metabolism: Cyproterone acetate is metabolized by various pathways, including hydroxylation and conjugation. The major metabolite in human plasma is the 15β-hydroxy derivative.
Elimination. Serum cyproterone acetate concentrations decline in a biphasic manner, with half-lives of 0.8 hours and 2.3 days. The serum clearance rate is approximately 3.6 ml/min-1/kg-1. Some of the steroid is excreted unchanged in the bile. The majority of the dose is excreted as metabolites in the urine and bile in a ratio of 3:7 with a half-life of 1.9 days. Metabolites are eliminated from plasma at a similar rate (half-life of 1.7 days).
Steady state. Accumulation of cyproterone acetate in the body during one course of treatment is quite expected, given the long half-life of elimination from serum in the terminal phase and its daily intake. The average maximum levels of cyproterone acetate in serum increase from 15 ng/ml (day 1) to 21 ng/ml and 24 ng/ml at the end of the first and third courses of treatment, respectively. The equilibrium concentration is reached after approximately 10 days. Given the long half-life of cyproterone acetate from serum, its accumulation in serum can be observed during one course of therapy with a factor of 2-2.5.
Smoking does not affect the pharmacokinetics of cyproterone acetate.
Ethinylestradiol
Adsorption: Ethinylestradiol is rapidly and completely absorbed after oral administration. After a single dose, the maximum serum concentration is approximately 80 pg/ml and is reached after 1.7 hours.
Distribution: For ethinylestradiol, the apparent volume of distribution was determined to be approximately
5 l/kg. Ethinylestradiol binds strongly, but nonspecifically, to serum albumin.
2% of the total level is present in an unbound form.
The bioavailability of ethinylestradiol can be altered in both directions by other active substances. However, there is no interaction with high doses of vitamin C. Ethinylestradiol, when administered continuously, induces hepatic synthesis of GH and corticosteroid-binding globulin (GBG). However, the degree of induction of GBG depends on the chemical structure and dose of the concomitant progestogen. During treatment with Diane-35, an increase in serum GH from about 100 nmol/l to 300 nmol/l and serum GH from about 50 pg/ml to 95 pg/ml was observed.
Metabolism: Metabolism of ethinylestradiol occurs during absorption and first-pass metabolism, resulting in reduced absolute and variable oral bioavailability. The metabolic clearance rate of ethinylestradiol from plasma has been estimated to be approximately 5 mL/min/kg.
In vitro, ethinylestradiol is a reversible inhibitor of CYP2C19, CYP1A1 and CYP1A2, and based on its mechanism of action, an inhibitor of CYP3A4/5, CYP2C8 and CYP2J2.
Elimination. The serum level of ethinylestradiol decreases in two phases with half-lives of 1-2 hours and about 20 hours, respectively. For analytical reasons, these parameters can only be calculated for high doses. The substance is not excreted unchanged, and ethinylestradiol metabolites are excreted in the urine and bile in a ratio of 4:6. The half-life of the metabolites is approximately 1 day.
Steady state. According to the half-life of ethinylestradiol from serum in the terminal phase and daily consumption of the substance, equilibrium concentrations are reached after 3-4 days and are 30-40% higher than those after a single dose.
Preclinical data
Ethinylestradiol
The toxicity profile of ethinylestradiol is well established. There are no preclinical safety data that would indicate relevant risks for humans and supplement the information already provided in other sections of the instructions for medical use of the drug.
Cyproterone acetate
According to preclinical studies, based on the results of studies on toxicity after repeated administration, no special risks for humans were identified when using Diane-35.
The use of cyproterone acetate during the hormone-sensitive phase of differentiation of the genital organs causes signs of feminization in male embryos at high doses. Observation of newborn males exposed to cyproterone acetate in utero has not revealed any signs of feminization. However, pregnancy is a contraindication to the use of Diane-35.
After treatment during the period of organogenesis (treatment was completed before the external genitalia were fully differentiated) to study the toxic effect on embryofetal development using the combination of both active ingredients of the drug, no potential signs of teratogenic effects exceeding the known effect on the differentiation of the genital organs in males were detected.
Cyproterone acetate showed no evidence of mutagenicity in first-line genotoxicity tests. However, in further studies, cyproterone acetate was shown to produce DNA adducts (and enhance DNA repair activity) in animal and human liver cells.
The aforementioned DNA adduct formation was observed at systemic exposures expected at the recommended dosage regimens of cyproterone acetate. After cyproterone acetate therapy in vivo, an increased incidence of focal, possibly precancerous, liver lesions was observed, in which changes in cellular enzyme activity were observed in female animals. The clinical significance of these findings has not yet been determined. Existing clinical data do not indicate an increased incidence of liver tumors in humans.
Animal studies of the carcinogenicity of cyproterone acetate have not revealed any results that are fundamentally different from those for other steroid hormones. However, it should be borne in mind that sex hormones can provoke the growth of certain hormone-dependent tissues and tumors.
Overall, the available data do not provide grounds for raising any objection to the use of Diane-35 in humans according to the instructions, within the specified therapeutic indications and at the recommended doses.
Indication
Treatment of moderate to severe androgen-dependent acne (with/without seborrhea) and/or hirsutism in women of reproductive age.
Diane-35 should only be used if topical or systemic antibiotic therapy for the treatment of acne is ineffective.
Since Diane-35 is also a hormonal contraceptive, this drug should not be used in combination with other hormonal contraceptives (see section "Contraindications").
Contraindication
Medicinal products containing estrogen/progestogen combinations should not be used in the presence of at least one of the conditions listed below. If any of these conditions appear for the first time during use of the medicinal products mentioned, their use should be discontinued immediately.
Concomitant use of another hormonal contraceptive (see section "Indications").
Current or past history of venous thrombotic/thromboembolic events (e.g. deep vein thrombosis, pulmonary embolism). Personal or family history of idiopathic venous thromboembolism (VTE) (with the family history being based on VTE in parents, siblings or relatives at a relatively young age). Current or past history of arterial thrombotic/thromboembolic events (e.g. myocardial infarction) or conditions such as angina pectoris and transient ischemic attack. Current or past history of acute cerebrovascular accident.
The presence of severe or multiple risk factors for the development of venous or arterial thrombosis (see section "Special warnings and precautions for use"), namely:
Diabetes mellitus with vascular complications; Severe arterial hypertension; Severe dyslipoproteinemia. Hereditary or acquired predisposition to venous or arterial thrombosis, including resistance to activated protein C (APC), antithrombin III deficiency, protein C deficiency, protein S deficiency, hyperhomocysteinemia, presence of antiphospholipid antibodies (anticardiolipin antibodies, lupus anticoagulant). Sickle cell anemia. Severe liver disease (also biliary system disorders such as Dubin-Johnson syndrome and Rotor syndrome), until liver function tests return to normal. Current or history of liver tumors (benign or malignant). Vaginal bleeding of unknown etiology. History of migraine with focal neurological symptoms.
Smoking (see section "Special precautions for use").
Known or suspected malignant tumors (e.g., genital or breast) dependent on steroid sex hormones. Idiopathic jaundice of pregnancy or severe pruritus of pregnancy, history of herpes of pregnancy, otosclerosis with worsening of the condition during previous pregnancies. Planning, known or suspected pregnancy. Breastfeeding. Hypersensitivity to the active substances or to any of the components of the drug.
Diane-35 should not be used to treat men.
The drug Diane-35 is contraindicated when used simultaneously with drugs containing ombitasvir/paritaprevir/ritonavir and dasabuvir (see sections “Special instructions” and “Interaction with other medicinal products and other types of interactions”).
Interaction with other medicinal products and other types of interactions
The information for the concomitant medicinal product should be consulted to identify potential interactions.
The effect of other medicines on Diane-35
Interaction with substances that induce the activity of microsomal enzymes is possible, and, as a result, increased clearance of sex hormones and the appearance of breakthrough bleeding and/or ineffectiveness of contraceptive protection.
Enzyme induction can be detected after a few days of treatment. Maximum enzyme induction is generally observed after a few weeks. After discontinuation of treatment, enzyme induction may persist for about 4 weeks.
Women receiving treatment with any of these drugs should use a barrier method of contraception in addition to taking Diane-35 during this period. The barrier method of contraception should be used during the period of taking the concomitant drugs and for 28 days afterwards. If the additional barrier method is still in use when the tablets in the Diane-35 package have run out, the tablets from the next Diane-35 package should be continued without the usual 7-day break.
Substances that increase the clearance of the drug Diane-35 (reduced effectiveness of Diane-35 due to enzymatic induction):
for example, barbiturates, rifampicin, antiepileptic drugs (such as barbexaclone, carbamazepine, phenytoin, primidone) and possibly oxcarbazepine, topiramate, felbamate, griseofulvin and drugs containing St. John's wort (Hypericum).
Substances with different effects on the clearance of the drug Diane-35
Concomitant use of Diane-35 with many HIV/HCV protease inhibitors and non-nucleoside reverse transcriptase inhibitors may result in an increase or decrease in plasma estrogen or progestogen concentrations. In some cases, these changes may be clinically significant.
Active substances that reduce the clearance of estrogen-progestogen combinations (enzyme inhibitors)
The clinical significance of the potential interaction with enzyme inhibitors remains unclear.
Concomitant use of strong CYP3A4 inhibitors may increase plasma concentrations of estrogens, progestins, or both.
Etoricoxib at doses of 60 to 120 mg/day has been shown to increase plasma concentrations of ethinyl estradiol by 1.4- to 1.6-fold, respectively, when co-administered with a combined hormonal contraceptive containing 0.035 mg ethinyl estradiol.
Estrogen/progestogen combinations such as Diane-35 may affect the metabolism of other drugs. Accordingly, either increased (e.g., cyclosporine) or decreased (e.g., lamotrigine) plasma and tissue concentrations may occur.
The need for antidiabetic medications may vary as a result of the effect on glucose tolerance.
Clinical data suggest that ethinylestradiol inhibits the clearance of CYP1A2 substrates, which in turn causes a slight (e.g., when using theophylline) or moderate (e.g., when using tizanidine) increase in their plasma concentrations.
Pharmacodynamic interactions
Concomitant use with medicinal products containing ombitasvir/paritaprevir/ritonavir and dasabuvir with or without ribavirin increases the risk of alanine aminotransferase (ALT) elevations (see sections 4.3 and 4.4).
Therefore, women using Diane-35 should temporarily switch to an alternative method of contraception (e.g. progestogen-only contraceptives or non-hormonal methods) before starting therapy with this combination of drugs. Diane-35 can be resumed 2 weeks after completing therapy with this combination.
Other types of interactions
Laboratory studies
The use of medicinal products such as Diane-35 may affect the results of certain laboratory tests. These include biochemical parameters of liver, thyroid, adrenal and renal function; plasma levels of proteins (transporters) (e.g. corticosteroid-binding globulin), lipids/lipoprotein fractions, and parameters of carbohydrate metabolism, coagulation and fibrinolysis. However, such changes usually do not exceed the normal range.
Diane-35 cannot be used together with an additional hormonal contraceptive; such medicines should be discontinued before starting treatment with Diane-35 (see section "Method of administration and dosage").
Application features
The active ingredients of Diane-35 are the progestogen cyproterone acetate and the estrogen ethinylestradiol, which are taken for 21 days each month. The composition of the drug is similar to combined oral contraceptives (COCs).
Duration of use
Improvement occurs after at least 3 months. The doctor should regularly determine the need for continued treatment (see section "Method of administration and dosage").
In the presence of any of the conditions/risk factors listed below, the benefits of using Diane-35 should be weighed against the possible risks, taking into account the individual characteristics of each patient, and discussed with the woman before she decides to use the drug. In the event of exacerbation, worsening or first appearance of any of the conditions or risk factors listed below, the woman is advised to consult a doctor. The doctor should decide whether to discontinue use of Diane-35.
Circulatory disorders
Women taking Diane-35 are at higher risk of developing VTE than those not taking the drug. The highest risk of VTE is in women during the first year of using Diane-35 or when resuming or switching to this drug after a break in taking the drug for at least 1 month. VTE can be fatal in 1-2% of cases.
Epidemiological studies have shown that the frequency of VTE in women taking Diane-35 is 1.5-2 times higher than in those using COCs containing levonorgestrel and may be the same as when using COCs containing desogestrel/gestodene/drospirenone.
Patients treated with Diane-35 may include women with an innate increased risk of developing cardiovascular disease, for example, with polycystic ovary syndrome.
Based on the results of epidemiological studies, a link has been found between the use of COCs and an increased risk of arterial thrombotic and thromboembolic diseases (myocardial infarction, transient ischemic attack).
In rare cases, thrombosis has been reported in other blood vessels, such as the arteries and veins of the liver, kidneys, mesenteric vessels, brain vessels or retina, in women using hormonal contraceptives.
Symptoms of venous or arterial thrombotic events or stroke may include: unusual unilateral pain and/or swelling of the lower extremities; sudden severe chest pain that may radiate to the left arm; sudden shortness of breath; sudden onset of cough; any unusual, severe, prolonged headache; sudden decrease or complete loss of vision; diplopia; speech disorder or aphasia; vertigo; loss of consciousness with or without partial epileptic seizure; weakness or pronounced sudden numbness of half or one part of the body; motor disorders; “acute” abdomen.
age (the risk increases with age); smoking (with heavy smoking, the risk increases with age, especially in women over 35 years of age. Women over 35 years of age are recommended to abstain from smoking if they wish to use Diane-35); a complicated family history (for example, cases of VTE in brothers or sisters or parents at a relatively young age). If there is a hereditary predisposition or there is a suspicion of it, it is recommended to consult a doctor before starting the use of any hormonal contraceptive; prolonged immobilization, radical surgery, any surgery on the lower extremities, significant injuries. In these cases, it is recommended to stop using the drug (in the case of a planned operation at least 4 weeks before it) and not to restart it earlier than 2 weeks after full recovery of mobility. If Diane-35 has not been discontinued prematurely, the issue of prescribing antithrombotic therapy should be considered; obesity (body mass index more than 30 kg/m2).
Factors that increase the risk of arterial thromboembolic events or cerebrovascular accident:
age (the risk increases with age); smoking (with heavy smoking, the risk increases with age, especially in women over 35 years of age. Women over 35 years of age are recommended to refrain from smoking if they wish to use the drug Diane-35); dyslipoproteinemia; obesity (body mass index more than 30 kg/m2); arterial hypertension; migraine; heart valve disease; atrial fibrillation; complicated family history (for example, cases of arterial thromboembolism in brothers or sisters or parents at a relatively young age). If there is a suspicion of a hereditary predisposition, it is recommended to consult a doctor before starting the use of any hormonal contraceptive.
Other diseases that may be associated with circulatory disorders include: diabetes mellitus, systemic lupus erythematosus, hemolytic uremic syndrome, chronic inflammatory bowel diseases (Crohn's disease or ulcerative colitis), and sickle cell anemia.
The increased risk of thromboembolism in the postpartum period should be taken into account (see section "Use during pregnancy or breastfeeding").
An increase in the frequency of migraine or its exacerbation during the use of Diane-35 (which may be a prodromal symptom of cerebrovascular accident) is one of the reasons for the possible immediate withdrawal of Diane-35.
Women taking Diane-35 should be advised to contact their doctor if they experience any symptoms of thrombosis. If thrombosis is suspected or confirmed, Diane-35 should be discontinued. Due to the teratogenic effects of anticoagulants (coumarins), appropriate contraceptive methods should be used.
Arterial thromboembolic complications can be life-threatening or fatal.
It should be borne in mind that the risk of thrombosis may increase due to the synergistic action of individual risk factors, if several such factors are present, or if the patient has any pronounced risk factor.
The drug Diane-35 should not be prescribed in case of negative results of the benefit/risk assessment (see section "Contraindications").
Tumors
The most important risk factor for cervical cancer is persistent human papillomavirus (HPV) infection. Some epidemiological studies have suggested that long-term use of estrogen-progestogen combinations may increase this risk, but this statement is still controversial, as it is not clear to what extent the studies took into account confounding factors, such as regularity of cervical screening and sexual behavior, including the use of barrier methods of contraception.
In isolated cases, benign and, even more rarely, malignant liver tumors have been observed in women using hormonal substances such as those contained in Diane-35, which in some cases led to life-threatening intra-abdominal bleeding. In the event of complaints of severe pain in the epigastric region, liver enlargement or signs of intra-abdominal bleeding, the possibility of a liver tumor should be considered in the differential diagnosis.
Malignant tumors can be life-threatening or fatal.
Other diseases
Women with hypertriglyceridemia or a family history of this condition may be at increased risk of developing pancreatitis when using estrogen-progestogen combinations.
Although a slight increase in blood pressure has been reported in many women taking estrogen-progestogen combinations (such as, for example, COCs or Diane-35), clinically significant increases are rare. If persistent clinically significant hypertension develops while taking Diane-35, the drug should be discontinued and treatment for the hypertension should be initiated. If blood pressure is normalized after antihypertensive therapy, Diane-35 can be resumed if deemed appropriate.
The following conditions have been reported to occur or worsen during pregnancy and with the use of estrogen-progestogen combinations, but their relationship to the use of estrogen-progestogen combinations has not been proven: cholestatic jaundice and/or pruritus, gallstone formation, porphyria, systemic lupus erythematosus, hemolytic-uremic syndrome, Sydenham's chorea, herpes gestationis, hearing loss associated with otosclerosis, epilepsy.
In women with hereditary angioedema, exogenous estrogens may induce or exacerbate symptoms of the disease.
Acute or chronic liver dysfunction may require discontinuation of Diane-35 until liver function tests return to normal. Recurrence of cholestatic jaundice and/or pruritus associated with cholestasis, which first occurred during pregnancy or previous use of sex steroids, also requires discontinuation of Diane-35.
Although estrogen-progestogen combinations may affect peripheral insulin resistance and glucose tolerance, there is no evidence to date that patients with diabetes mellitus using low-dose estrogen-progestogen combinations (containing < 0.05 mg ethinylestradiol) require any change in their therapeutic regimen. However, diabetic patients should be closely monitored while using Diane-35.
Crohn's disease and ulcerative colitis are associated with the use of estrogen-progestogen combinations.
Depressed mood and depression are well-known side effects that can occur with hormonal contraceptives (see section 4.8). Depression can be a serious condition and is a well-known risk factor for suicidal behaviour and suicide. Women should be advised to seek medical advice if they experience mood changes or symptoms of depression, including shortly after starting treatment.
Chloasma may occasionally occur, especially in women with a history of chloasma during pregnancy. Women with a tendency to chloasma should avoid exposure to the sun or ultraviolet radiation while using Diane-35.
Elevated ALT levels
In clinical trials in patients treated for hepatitis C with the medicinal products containing ombitasvir/paritaprevir/ritonavir and dasabuvir with or without ribavirin, transaminase (ALT) elevations greater than 5 times the upper limit of normal were observed significantly more frequently in women using medicinal products containing ethinylestradiol, such as combined hormonal contraceptives or Diane-35 (see sections 4.3 and 4.5).
Decreased efficiency
The contraceptive efficacy of Diane-35 may be reduced in the event of missed tablets (see section "Method of administration and dosage"), gastrointestinal disorders (see section "Method of administration and dosage") or when used simultaneously with other medicines (see section "Interaction with other medicines and other types of interactions").
Irregular bleeding
When using all medicines containing a combination of estrogen/progestogen, irregular bleeding (spotting or breakthrough bleeding) may occur, especially during the first months of use.
1 tablet of the medicinal product contains 31 mg of lactose monohydrate and 19 mg of sucrose.
Patients with rare hereditary problems of galactose or fructose intolerance, lactase deficiency, glucose-galactose malabsorption, or sucrase-isomaltase deficiency should not use Diane-35.
Ability to influence reaction speed when driving vehicles or other mechanisms
In patients taking Diane-35, there was no effect of the drug on the ability to drive or operate machinery.
Use during pregnancy or breastfeeding
Before starting the drug, pregnancy must be excluded. The drug is contraindicated during pregnancy. If pregnancy occurs during the use of Diane-35, its use must be stopped immediately, but this is not a reason for termination of pregnancy.
The drug is contraindicated during breastfeeding. Cyproterone acetate is excreted in breast milk. About 0.2% of the maternal dose may enter the body of a breastfed infant, which corresponds to approximately 1 μg/kg.
During breastfeeding, about 0.02% of the daily maternal dose of ethinylestradiol may enter the infant's body through breast milk.
Method of administration and doses
Diane-35 inhibits ovulation and thus exerts a contraceptive effect. Therefore, patients receiving treatment with Diane-35 should not use additional hormonal contraceptives, as this would result in an overdose of hormones and is not necessary to ensure effective contraceptive protection. For the same reason, women who wish to become pregnant should not take Diane-35. To achieve the proper therapeutic effect and contraceptive protection, Diane-35 must be taken regularly.
Method of application
For oral use.
Dosage
The tablets should be taken daily in the order indicated on the blister, at approximately the same time, with a small amount of liquid. The drug is taken by
1 tablet per day for 21 consecutive days. Taking the tablets from each subsequent package should be started after a 7-day break in taking the drug, during which menstrual-like bleeding usually occurs, which usually begins on the 2-3rd day after taking the last tablet and may continue until the start of taking the tablets from a new package.
Contraceptive protection begins on the first day of tablet-taking and continues during the 7-day tablet-free period. Therefore, simultaneous use of hormonal contraceptives should be discontinued.
Medical examination
Before starting the drug, it is recommended to conduct a thorough general medical examination (including measurement of body weight, blood pressure, examination of the cardiovascular system, condition of the lower extremities and skin, urine analysis for glucose and acetone, and examination of the hepatobiliary system, if necessary), a gynecological examination (including examination of the mammary glands and cytological examination of the cervix (it is advisable to obtain material from the surface of the vaginal part of the cervix and from the walls of the cervical canal)), as well as to collect a detailed family history in order to identify diseases requiring treatment and determine existing risks. Pregnancy should be excluded. During the use of the drug, it is recommended to conduct an examination once every 6 months.
If close relatives have had thromboembolic events at a young age (e.g. deep vein thrombosis, stroke, myocardial infarction), the possibility of a blood clotting disorder should be ruled out.
It is also necessary to draw women's attention to the fact that the use of oral contraceptives does not protect against HIV infections (AIDS) and other sexually transmitted diseases.
Starting to use Diane-35
If hormonal contraceptives
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.