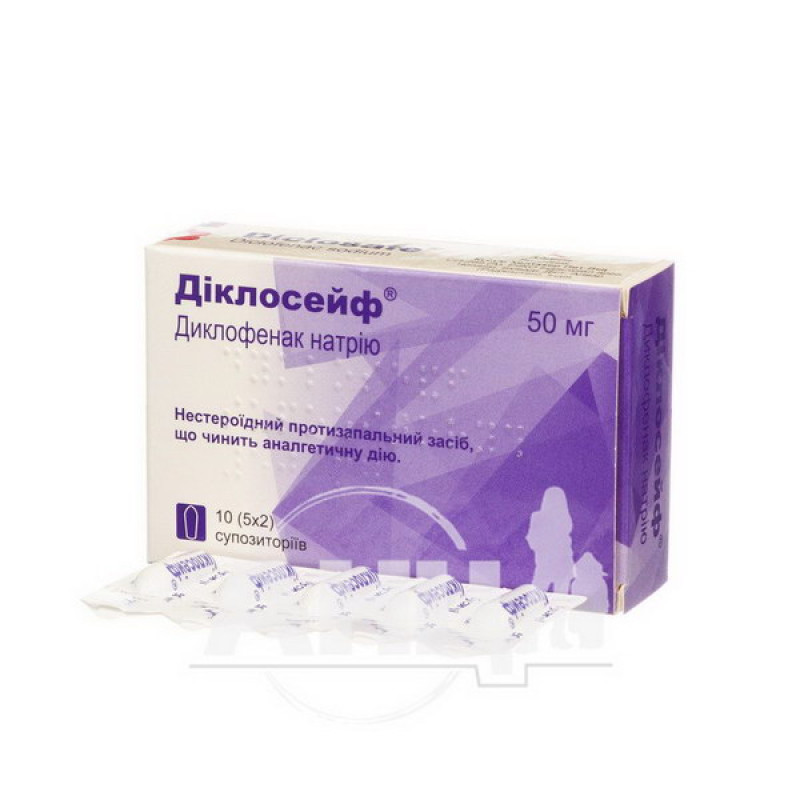
Dicloceif suppositories 50 mg strip No. 10

Instructions for Dicloceif suppositories 50 mg strip No. 10
Composition
active ingredient: diclofenac;
1 suppository contains diclofenac sodium 50 mg;
excipient: solid fat.
Dosage form
Suppositories.
Main physicochemical properties: suppositories from white to light yellow in color, torpedo-shaped.
Pharmacotherapeutic group
Nonsteroidal anti-inflammatory and antirheumatic drugs.
ATX code M01A B05.
Pharmacological properties
Pharmacodynamics
Diclofenac sodium is a nonsteroidal anti-inflammatory drug that has a pronounced analgesic and anti-inflammatory effect. It is an inhibitor of prostaglandin synthetase (cyclooxygenase).
Pharmacokinetics
Absorption.
Absorption is rapid, but slower than with enteric-coated tablets.
After administration of 50 mg diclofenac sodium suppositories, the concentration per unit dose is about 2/3 of the concentration achieved after administration of enteric-coated tablets (1.95 + 0.8 μg/ml (1.9 μg/ml = 5.9 μmol/l)).
Bioavailability.
As with oral dosage forms, the area under the concentration curve (AUC) is approximately half that obtained with parenteral dosing. After repeated administration, the pharmacokinetics of the drug do not change. Cumulation of the drug is not observed when the recommended dosage is observed.
Distribution.
The binding of diclofenac to serum proteins is 99.7%, mainly to albumin - 99.4%.
Diclofenac penetrates into the synovial fluid, where its maximum concentration is reached 2-4 hours later than in the blood plasma. The apparent half-life from the synovial fluid is 3-6 hours. 2 hours after reaching maximum plasma concentrations, the concentration of diclofenac in the synovial fluid remains higher than in the blood plasma; this phenomenon is observed for 12 hours.
Diclofenac was detected in low concentrations (100 ng/ml) in breast milk from one woman. The estimated amount of drug reaching the infant in breast milk is equivalent to a dose of 0.03 mg/kg/day.
Metabolism.
Diclofenac is metabolized partly by glucuronidation of the unchanged molecule, but mainly by single and multiple hydroxylation and methoxylation, leading to the formation of several phenolic metabolites, most of which form conjugates with glucuronic acid. Two of these phenolic metabolites are biologically active, but much less so than diclofenac.
Breeding.
The total systemic clearance of diclofenac is 263 ± 56 ml/min (mean + SD). The terminal plasma half-life is 1-2 hours. The plasma half-life of four metabolites, including two pharmacologically active ones, is also short and is 1-3 hours. About 60% of the administered dose of the drug is excreted in the urine as a glucuronide conjugate of the intact molecule and as metabolites, most of which are also converted to glucuronide conjugates. Less than 1% of diclofenac is excreted unchanged. The remainder of the administered dose of the drug is excreted as metabolites in the feces.
Pharmacokinetics in certain groups of patients.
No effect of patient age on absorption, metabolism, or elimination was observed, except that in five elderly patients, a 15-minute intravenous infusion resulted in plasma concentrations that were 50% higher than expected in young healthy volunteers.
In patients with renal impairment receiving therapeutic doses, no accumulation of unchanged active substance is expected based on the kinetics of the drug after a single dose. In patients with creatinine clearance less than 10 ml/min, the estimated steady-state plasma concentrations of hydroxylated metabolites were approximately 4-fold higher than in healthy volunteers. However, all metabolites were ultimately excreted in the bile.
Patients with liver dysfunction.
In patients with chronic hepatitis or compensated cirrhosis, the pharmacokinetics and metabolism of diclofenac are similar to those in patients without liver disease.
Indication
inflammatory and degenerative forms of rheumatism: rheumatoid arthritis, juvenile rheumatoid arthritis, ankylosing spondylitis, osteoarthritis, including spondyloarthritis; pain syndromes of the spine; rheumatic diseases of extra-articular soft tissues; post-traumatic and postoperative pain syndromes accompanied by inflammation and edema, in particular after dental and orthopedic operations; gynecological diseases accompanied by pain syndrome and inflammation, such as primary dysmenorrhea and adnexitis; migraine attacks; acute attacks of gout; as an adjuvant in severe inflammatory diseases of the ENT organs accompanied by pain, such as pharyngotonsillitis, otitis; in accordance with general therapeutic principles, the underlying disease should be treated with basic therapy. Fever itself is not an indication for the use of the drug.
Contraindication
Hypersensitivity to the active substance or to any of the excipients of the drug; history of gastrointestinal bleeding or perforation associated with previous treatment with nonsteroidal anti-inflammatory drugs (NSAIDs); active ulcer/bleeding or history of recurrent ulcer/bleeding (two or more separate episodes of established ulcer or bleeding); 3rd trimester of pregnancy; inflammatory bowel disease (e.g. Crohn's disease or ulcerative colitis); hepatic failure; renal failure; congestive heart failure (NYHA II-IV), ischemic heart disease in patients with angina pectoris, myocardial infarction; treatment of perioperative pain in coronary artery bypass grafting (or use of an artificial blood circulation device); cerebrovascular disease in patients with stroke or transient ischemic attacks; peripheral arterial disease; proctitis; Diclofenac sodium, like other NSAIDs, is contraindicated in patients who develop attacks of bronchial asthma, urticaria, angioedema or acute rhinitis in response to taking acetylsalicylic acid or other NSAIDs.
Interaction with other medicinal products and other types of interactions
Interactions observed when using diclofenac in the form of enteric-coated tablets and/or in other dosage forms are indicated.
Lithium: Diclofenac may increase plasma lithium concentrations when used concomitantly. Monitoring of serum lithium levels is recommended.
Digoxin: Diclofenac may increase the plasma concentration of digoxin when used concomitantly. Monitoring of serum digoxin levels is recommended.
Diuretics and antihypertensives: As with other NSAIDs, concomitant use of diclofenac with diuretics and antihypertensives (e.g. β-blockers, angiotensin-converting enzyme (ACE) inhibitors) may lead to a reduction in their antihypertensive effect by inhibiting the synthesis of vasodilator prostaglandins. Therefore, such a combination should be used with caution and patients, especially the elderly, should be closely monitored for blood pressure. Patients should be adequately hydrated and monitoring of renal function is recommended after initiation of concomitant therapy and regularly thereafter, especially with diuretics and ACE inhibitors, due to the increased risk of nephrotoxicity.
Drugs known to cause hyperkalemia.
Concomitant treatment with potassium-sparing diuretics, cyclosporine, tacrolimus or trimethoprim may be associated with increases in serum potassium levels, therefore patients should be monitored more frequently.
Anticoagulants and antiplatelet agents. Concomitant use may increase the risk of bleeding, therefore caution is advised. Although clinical studies do not indicate an effect of diclofenac on the activity of anticoagulants, there is some evidence of an increased risk of bleeding in patients receiving diclofenac and anticoagulants concomitantly. Therefore, close monitoring of such patients is recommended to ensure that no changes in the dosage of anticoagulants are necessary. As with other NSAIDs, diclofenac in high doses may transiently inhibit platelet aggregation.
Other NSAIDs, including selective cyclooxygenase-2 inhibitors and corticosteroids. Concomitant use of diclofenac and other NSAIDs or corticosteroids may increase the risk of gastrointestinal bleeding or ulceration. The concomitant use of two or more NSAIDs should be avoided.
Selective serotonin reuptake inhibitors (SSRIs): Concomitant use of NSAIDs and SSRIs may increase the risk of gastrointestinal bleeding.
Antidiabetic drugs. Clinical studies have shown that diclofenac can be used together with oral antidiabetic drugs without changing their therapeutic effect. However, there are some reports of the development of both hypoglycemia and hyperglycemia in such cases, which necessitated the need to change the dose of antidiabetic drugs during the use of diclofenac. Therefore, it is recommended to monitor blood glucose levels during combination therapy.
There have also been isolated reports of metabolic acidosis with concomitant use with diclofenac, especially in patients with pre-existing renal impairment.
Methotrexate. Diclofenac may inhibit the renal tubular clearance of methotrexate, leading to increased methotrexate levels. Caution should be exercised when NSAIDs, including diclofenac, are administered less than 24 hours before methotrexate, as this may increase the blood concentration of methotrexate and increase its toxicity. Serious toxicity has been reported when the interval between administration of methotrexate and NSAIDs, including diclofenac, was within 24 hours. This interaction is mediated by accumulation of methotrexate as a result of impaired renal excretion in the presence of NSAIDs.
Tacrolimus: There is a possible increased risk of nephrotoxicity when NSAIDs are used with tacrolimus, which may be mediated through the renal antiprostaglandin effects of the NSAID and the calcineurin inhibitor.
Quinolone antibacterials. There are isolated reports of seizures in patients receiving concomitant quinolones and NSAIDs. This may occur in patients with or without a history of epilepsy and seizures. Therefore, caution should be exercised when considering the use of a quinolone in patients already receiving NSAIDs.
Phenytoin: When phenytoin is used concomitantly with diclofenac, monitoring of phenytoin plasma concentrations is recommended due to the expected increase in phenytoin exposure.
Colestipol and cholestyramine. These drugs may delay or reduce the absorption of diclofenac. Therefore, it is recommended to administer diclofenac at least 1 hour before or 4-6 hours after colestipol/cholestyramine.
Cardiac glycosides: Concomitant use of cardiac glycosides and NSAIDs in patients may exacerbate heart failure, reduce glomerular filtration rate, and increase plasma levels of cardiac glycosides.
Mifepristone: NSAIDs should not be used for 8-12 days after mifepristone administration, as NSAIDs may reduce the effect of mifepristone.
CYP2C9 inhibitors: Caution is advised when diclofenac is co-administered with CYP2C9 inhibitors (e.g. voriconazole). This may lead to a significant increase in maximum plasma concentrations and exposure to diclofenac.
CYP2C9 inducers: Caution is advised when co-administering diclofenac with CYP2C9 inducers (e.g. rifampicin). This may lead to a significant increase in plasma concentrations and exposure to diclofenac.
Application features
General.
To minimize unwanted effects, the lowest effective dose should be used for the shortest period of time.
The concomitant use of Dicloceif with systemic NSAIDs such as selective cyclooxygenase-2 inhibitors should be avoided due to the lack of any evidence of a synergistic effect and the association with potential additive side effects.
Caution is required when using the drug in patients over 65 years of age. In particular, it is recommended to use the lowest effective dose in frail elderly patients or those with low body weight.
In rare cases, as with other NSAIDs, allergic reactions, including anaphylactic/anaphylactoid reactions, may occur, even without prior exposure to diclofenac. Due to its pharmacodynamic properties, Diclofenac, like other NSAIDs, may mask the signs and symptoms of infection.
Effect on the digestive tract.
Gastrointestinal bleeding (hemorrhage, melena), ulceration or perforation, which can be fatal and may occur at any time during treatment, with or without warning symptoms or a previous history of serious gastrointestinal events, have been reported with all NSAIDs, including diclofenac. These events are usually more severe in the elderly. If gastrointestinal bleeding or ulceration occurs in patients receiving diclofenac sodium, the drug should be discontinued.
As with other NSAIDs, including diclofenac, medical supervision and special caution are required in patients presenting with symptoms suggestive of gastrointestinal (GI) disorders. The risk of GI bleeding, ulceration or perforation increases with increasing dose of NSAIDs, including diclofenac, and in patients with a history of ulcer, especially complicated by bleeding or perforation, and in the elderly.
Elderly patients have an increased frequency of adverse reactions to NSAIDs, especially gastrointestinal bleeding and perforation, which can be fatal.
To reduce the risk of such toxic effects on TT, treatment should be initiated and maintained at the lowest effective dose.
For such patients, as well as those requiring concomitant low-dose acetylsalicylic acid (ASA/aspirin or other drugs that may increase the risk of adverse effects on the gastrointestinal tract), combination therapy with protective agents (e.g., proton pump inhibitors or misoprostol) should be considered. Patients with a history of gastrointestinal toxicity, especially the elderly, should be advised to report any unusual abdominal symptoms (especially gastrointestinal bleeding). Caution is also warranted in patients receiving concomitant medications that may increase the risk of ulceration or bleeding, such as systemic corticosteroids, anticoagulants (e.g., warfarin), antithrombotic agents (e.g., acetylsalicylic acid), or C/CZS.
Close medical supervision and caution are required in patients with ulcerative colitis or Crohn's disease, as these conditions may be exacerbated.
Effect on the liver.
As with other NSAIDs, including diclofenac, one or more liver enzymes may increase. During prolonged use of diclofenac sodium, regular monitoring of liver function should be performed as a precautionary measure. If liver function abnormalities persist or worsen, and if clinical signs or symptoms suggestive of progressive liver disease or if other manifestations occur (e.g. eosinophilia, rash), Diclofenac should be discontinued. Diseases such as hepatitis may occur without prodromal symptoms. Caution is advised when Diclofenac is used in patients with hepatic porphyria due to the possibility of triggering an attack of porphyria.
Effect on the kidneys.
Since cases of fluid retention and edema have been reported with NSAIDs, including diclofenac, special attention should be paid to patients with impaired cardiac or renal function, a history of hypertension, elderly patients, patients receiving concomitant therapy with diuretics or drugs that significantly affect renal function, and patients with a significant decrease in extracellular fluid volume for any reason, such as before or after major surgery. In such cases, monitoring of renal function is recommended as a precautionary measure. Discontinuation of therapy usually leads to a return to the state that existed before treatment.
Effect on the skin.
Serious skin reactions (some of which were fatal) have been reported very rarely with the use of NSAIDs, including diclofenac sodium. Patients are at greatest risk of developing these reactions at the beginning of therapy: the onset of the reaction is noted in most cases within the first month of treatment. Diclofenac should be discontinued at the first appearance of skin rash, mucosal lesions or any other sign of hypersensitivity.
Systemic lupus erythematosus and mixed connective tissue diseases.
Patients with systemic lupus erythematosus (SLE) and mixed connective tissue diseases may be at increased risk of developing aseptic meningitis.
Cardiovascular and cerebrovascular effects.
Diclofenac should only be prescribed to patients with significant risk factors for cardiovascular events (e.g. hypertension, hyperlipidemia, diabetes mellitus, smoking) after careful clinical evaluation. Since the cardiovascular risks of diclofenac may increase with increasing dose and duration of treatment, it should be used for the shortest possible period and at the lowest effective dose. The patient's need for diclofenac for symptom relief and response to therapy should be reassessed periodically.
For patients with a history of hypertension and/or mild to moderate congestive heart failure, appropriate monitoring and advice is necessary, as fluid retention and oedema have been reported in association with the use of NSAIDs, including diclofenac.
Clinical trial data and epidemiological data suggest that the use of diclofenac, especially at high doses (150 mg/day) and with long-term treatment, may be associated with a small increased risk of arterial thrombotic events (e.g. myocardial infarction or stroke).
Patients with uncontrolled hypertension, congestive heart failure, ischemic heart disease, peripheral arterial disease should use diclofenac only after careful consultation.
Effect on hematological parameters.
With prolonged use of diclofenac sodium, as with other NSAIDs, monitoring of complete blood counts is recommended.
Diclofenac may reversibly inhibit platelet aggregation. Patients with impaired hemostasis, hemorrhagic diathesis or hematological disorders should be carefully monitored.
History of bronchial asthma.
Patients with bronchial asthma, seasonal allergic rhinitis, nasal mucosal edema (i.e. nasal polyps), chronic obstructive pulmonary disease or chronic respiratory tract infections (especially those associated with allergic rhinitis-like symptoms) are more likely to experience reactions to NSAIDs, such as exacerbation of bronchial asthma (so-called analgesic intolerance/analgesic asthma), angioedema or urticaria. Therefore, special precautions (emergency medical attention) are recommended for such patients. This also applies to patients with allergic reactions to other substances, such as rash, itching or urticaria.
Like other drugs that inhibit prostaglandin synthetase activity, diclofenac sodium and other NSAIDs may provoke the development of bronchospasm when used in patients suffering from bronchial asthma or in patients with a history of bronchial asthma.
Ability to influence reaction speed when driving vehicles or other mechanisms
Patients who experience visual disturbances, dizziness, vertigo, drowsiness, central nervous system disorders, lethargy or increased fatigue during diclofenac sodium therapy should not drive or operate complex machinery.
Use during pregnancy or breastfeeding
Pregnancy.
In the first and second trimesters of pregnancy, diclofenac sodium should be used only if the expected benefit to the mother outweighs the potential risk to the fetus and only at the lowest effective dose, and the duration of treatment should be as short as possible. As with other NSAIDs, the drug is contraindicated in the last 3 months of pregnancy (possible suppression of uterine contractility and premature closure of the ductus arteriosus in the fetus). Inhibition of prostaglandin synthesis may adversely affect pregnancy and/or embryo/fetal development. Epidemiological studies have shown an increased risk of miscarriage and/or the risk of heart defects and gastroschisis after use of a prostaglandin synthesis inhibitor in early pregnancy. The absolute risk of cardiovascular defects was increased from less than 1% to approximately 1.5%. It is possible that the risk increases with dose and duration of treatment. It has been shown that in animals, administration of a prostaglandin synthesis inhibitor results in increased pre- and post-implantation loss and embryo/fetal lethality.
In addition, in animals treated with a prostaglandin synthesis inhibitor during organogenesis, an increased incidence of various malformations, including cardiovascular, has been reported. If diclofenac sodium is used by a woman attempting to conceive, or during the first trimester of pregnancy, the dose should be kept as low and the duration of treatment as short as possible.
During the third trimester of pregnancy, the following undesirable effects may occur when using prostaglandin synthesis inhibitors:
Cardiopulmonary toxicity (with premature closure of the ductus arteriosus and pulmonary hypertension); renal dysfunction, which may progress to renal failure with oligohydramnios.
In the mother and newborn, as well as at the end of pregnancy:
Possible prolongation of bleeding time, antiplatelet effect, which can be observed even at very low doses; inhibition of uterine contractions, leading to delayed or prolonged labor.
Therefore, diclofenac sodium is contraindicated in the third trimester of pregnancy.
Breastfeeding period.
Like other NSAIDs, diclofenac passes into breast milk in small amounts. In this regard, diclofenac suppositories should not be used by women during breastfeeding to avoid undesirable effects on the infant.
Female fertility.
As with other NSAIDs, diclofenac sodium may impair female fertility and is therefore not recommended for use in women attempting to conceive. In women who have difficulty conceiving or are undergoing investigation of infertility, discontinuation of diclofenac sodium should be considered.
Method of administration and doses
The minimum effective dose should be used for the shortest period of time, taking into account the treatment objectives for each individual patient. Do not take orally, for rectal administration only. Suppositories should be inserted into the rectum as deeply as possible, preferably after bowel cleansing. Suppositories should not be divided into parts, as such a change in the method of administration of the drug may lead to a violation of the distribution of the active substance.
Adults: The initial dose is usually 100-150 mg per day. For mild symptoms and long-term therapy, a dose of 75*-100 mg per day is sufficient.
Divide the daily dose into 2-3 doses. To avoid night pain or morning stiffness, administer diclofenac sodium in the form of rectal suppositories before bedtime before taking the drug during the day (the daily dose should not exceed 150 mg).
In primary dysmenorrhea, the daily dose should be selected individually, usually it is 50-150 mg per day. The initial dose may be 50-100 mg per day, but if necessary it can be increased over several menstrual cycles to a maximum of 200 mg per day.
The drug should be started after the first pain symptoms appear and continued for several days, depending on the dynamics of symptom regression.
For the treatment of migraine attacks, the course should be started with a dose of 100* mg at the first signs of the onset of an attack. If necessary, another 100* mg of diclofenac can be used on the same day. If necessary, the treatment can be continued on the following days (the daily dose of the drug should not exceed 150 mg, the dose should be divided into 2-3 doses).
* Use in appropriate dosage.
Children (age 14 and older): 50 mg suppositories may be prescribed.
Elderly patients: Although the pharmacokinetics of diclofenac sodium are not altered to any clinically significant extent in elderly patients, NSAIDs should be used with caution in such patients, as they are generally more susceptible to adverse reactions. In particular, the lowest effective dose is recommended for elderly patients who are debilitated or have low body mass; patients should also be monitored for gastrointestinal bleeding when receiving NSAIDs.
Diclofenac is contraindicated in patients with severe renal impairment (see section 4.3). No specific studies have been conducted in patients with renal impairment, so there is no reason to believe that patients with renal impairment require special dose adjustment. Caution is recommended when taking diclofenac in patients with mild to moderate renal impairment (see section 4.4).
Patients with liver dysfunction.
Diclofenac is contraindicated in patients with severe hepatic impairment (see section 4.4). No specific studies have been conducted in patients with hepatic impairment, and there is no reason to believe that patients with hepatic impairment require special dose adjustment. Caution is advised when diclofenac is administered to patients with mild to moderate hepatic impairment (see section 4.4).
Children
Dicloceif, 50 mg suppositories, can be used in children from 14 years of age.
Overdose
Symptoms. There is no typical clinical picture characteristic of diclofenac overdose. Overdose may cause symptoms such as headache, nausea, vomiting, epigastric pain, gastrointestinal bleeding, diarrhea, dizziness, disorientation, agitation, coma, drowsiness, tinnitus and convulsions. Acute renal failure and liver damage are possible in case of severe intoxication.
Treatment. If necessary, symptomatic treatment. Activated charcoal should be considered within 1 hour of ingestion of a potentially toxic amount of the drug. In addition, gastric lavage should be considered for adult patients within 1 hour of ingestion of a potentially toxic amount of the drug. Diazepam should be administered intravenously in case of frequent or prolonged seizures. Other measures may be indicated depending on the clinical condition of the patient.
Adverse reactions
From the blood and lymphatic system: thrombocytopenia, leukopenia, anemia (hemolytic anemia, aplastic anemia), agranulocytosis.
Immune system disorders: hypersensitivity, anaphylactic and anaphylactoid reactions (including hypotension and shock), angioedema (including facial swelling).
Mental disorders: disorientation, depression, insomnia, irritability, nightmares, psychotic disorders.
From the nervous system: headache, dizziness, drowsiness, increased fatigue, paresthesia, memory impairment, convulsions, anxiety, tremor, aseptic meningitis, taste disorders, stroke, confusion, hallucinations, sensory disturbances, general malaise.
From the organs of vision: visual disturbances, blurred vision, diplopia, optic neuritis.
From the side of the organs of hearing and labyrinth of the ear: vertigo, tinnitus, hearing disorders.
Cardiovascular system: palpitations, chest pain, heart failure, myocardial infarction, hypertension, hypotension, vasculitis.
Respiratory, thoracic and mediastinal disorders: asthma (including dyspnea), pneumonitis.
Gastrointestinal: nausea, vomiting, diarrhea, dyspepsia, epigastric pain, flatulence, anorexia, gastritis, gastrointestinal bleeding, hematemesis, melena, hemorrhagic diarrhea, gastric and intestinal ulcers, with or without bleeding, gastrointestinal stenosis or perforation (sometimes fatal, especially in elderly patients), which can lead to peritonitis, colitis (including hemorrhagic colitis, ischemic colitis and exacerbation of ulcerative colitis or Crohn's disease), constipation, stomatitis (including ulcerative stomatitis), glossitis, esophageal dysfunction, diaphragmatic stenosis of the intestine, pancreatitis.
Hepatobiliary system: increased transaminase levels, hepatitis, jaundice, liver disorders, fulminant hepatitis, liver necrosis, liver failure.
Skin and subcutaneous tissue disorders: rash, urticaria, blistering rash, eczema, erythema, erythema multiforme, Stevens-Johnson syndrome, Lyell's syndrome (toxic epidermal necrolysis), exfoliative dermatitis, hair loss, photosensitivity reactions, purpura, including allergic, itching.
Renal and urinary disorders: acute kidney injury (acute renal failure), hematuria, proteinuria, interstitial nephritis, nephrotic syndrome, renal papillary necrosis.
General disorders and administration site conditions: injection site irritation, swelling, exacerbation of hemorrhoids.
Reproductive system and breast disorders: impotence.
Clinical trials and epidemiological data suggest an increased risk of thrombotic complications (e.g. myocardial infarction or stroke) associated with the use of diclofenac, particularly at high therapeutic doses (150 mg per day) and with long-term use.
Visual disturbances such as visual disturbances, blurred vision and diplopia are class effects of NSAIDs and are usually reversible upon discontinuation of the drug. The most likely mechanism of visual disturbances is inhibition of the synthesis of prostaglandins and other related compounds, which, by disrupting the regulation of retinal blood flow, contribute to the development of visual disturbances. If such symptoms occur during treatment with diclofenac, an ophthalmological examination should be performed to exclude other possible causes.
Expiration date
3 years.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 °C.
Keep out of reach of children.
Packaging
5 suppositories in a strip. 2 strips in a carton.
Vacation category
According to the recipe.
Producer
Kusum Healthcare Pvt Ltd.
Location of the manufacturer and its business address
SP-289 (A), RIICO Industrial Area, Chopanki, Bhiwadi, Dist. Alwar (Rajasthan), India.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.