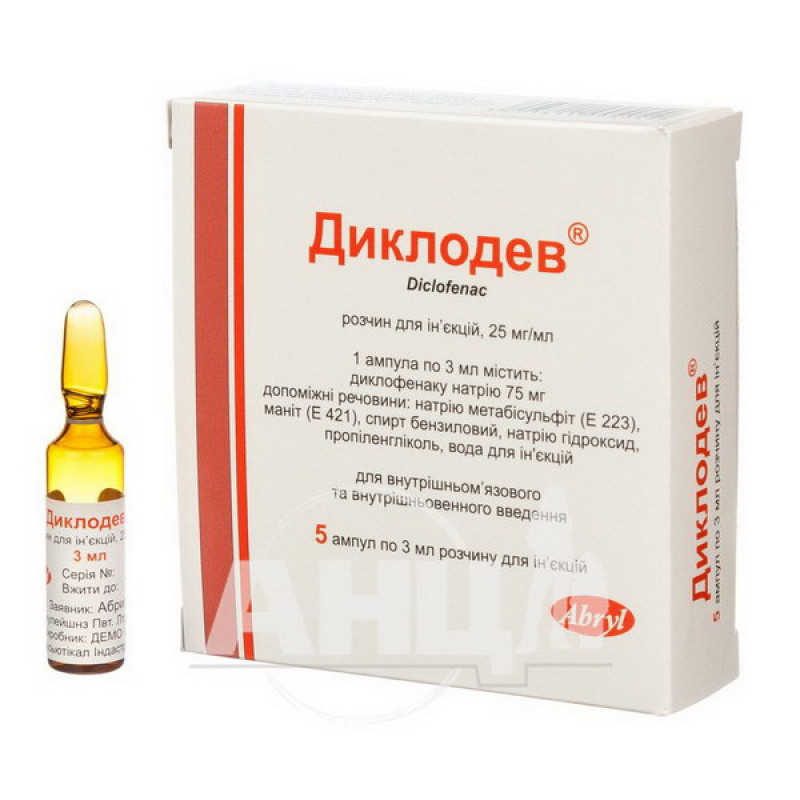
Diclodev solution for injection 25 mg/ml ampoule 3 ml No. 5
The drug Diclodev when administered is intended for the treatment of:
inflammatory and degenerative forms of rheumatism, rheumatoid arthritis, ankylosing spondylitis, osteoarthritis, spondyloarthritis, vertebral pain syndrome, non-articular rheumatism; acute attacks of gout; renal and biliary colic; pain and swelling after injuries and operations; severe migraine attacks.The drug, when administered as an intravenous infusion, is intended for the treatment or prevention of postoperative pain.
Composition
Active ingredient: diclofenac;
3 ml of solution contain 75 mg of diclofenac sodium (25 mg/ml);
Excipients: sodium metabisulfite (E 223), mannitol (E 421), benzyl alcohol, sodium hydroxide, propylene glycol, water for injections
Contraindication
Hypersensitivity to the active substance, sodium metabisulfite or to any other components of the drug.
History of gastrointestinal bleeding or perforation associated with previous treatment with nonsteroidal anti-inflammatory drugs (NSAIDs).
Active peptic ulcer/bleeding or history of recurrent peptic ulcer/bleeding (two or more separate episodes of established ulceration or bleeding).
Method of application
The drug should be used in the most effective doses for the shortest period of time, taking into account the treatment objectives of each individual patient.
The solution for injection should not be administered as a bolus injection.
Application features
Prescribe only if the expected benefit to the mother outweighs the potential risk to the fetus.
Children
Not applicable.
Drivers
You should not drive or operate complex machinery.
Overdose
There is no typical clinical picture of diclofenac overdose. Overdose may cause symptoms such as headache, nausea, vomiting, epigastric pain, gastrointestinal bleeding, diarrhea, dizziness, disorientation, agitation, coma, drowsiness, tinnitus, or convulsions. Acute renal failure and liver damage are possible in severe intoxication.
Activated charcoal may be used after ingestion of potentially toxic doses, and gastric lavage (e.g., induction of vomiting, gastric lavage) may be performed after ingestion of potentially life-threatening doses.
Side effects
Nervous system: often - headache, dizziness. Hearing and labyrinth disorders: often - vertigo. Gastrointestinal tract: often - nausea, vomiting, diarrhea, dyspepsia, abdominal pain, flatulence. Skin and subcutaneous tissue disorders: often - rash. Kidneys and urinary system: often - fluid retention in the body, edema.Interaction
Colestipol and cholestyramine. These drugs may delay or reduce the absorption of diclofenac. Therefore, it is recommended to administer diclofenac at least one hour before or 4-6 hours after colestipol/cholestyramine.
Cardiac glycosides. Concomitant use of cardiac glycosides and nonsteroidal anti-inflammatory drugs may exacerbate heart failure, reduce GFR, and increase plasma glycoside levels.
Mifepristone: NSAIDs should not be used for 8-12 days after mifepristone administration, as NSAIDs may reduce the effect of mifepristone.
Storage conditions
Store at a temperature not exceeding 25 °C in the original packaging.
Keep out of reach of children.
Shelf life - 3 years.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.