Difenda film-coated tablets 3 mg/0.02 mg blister (24+4) No. 28
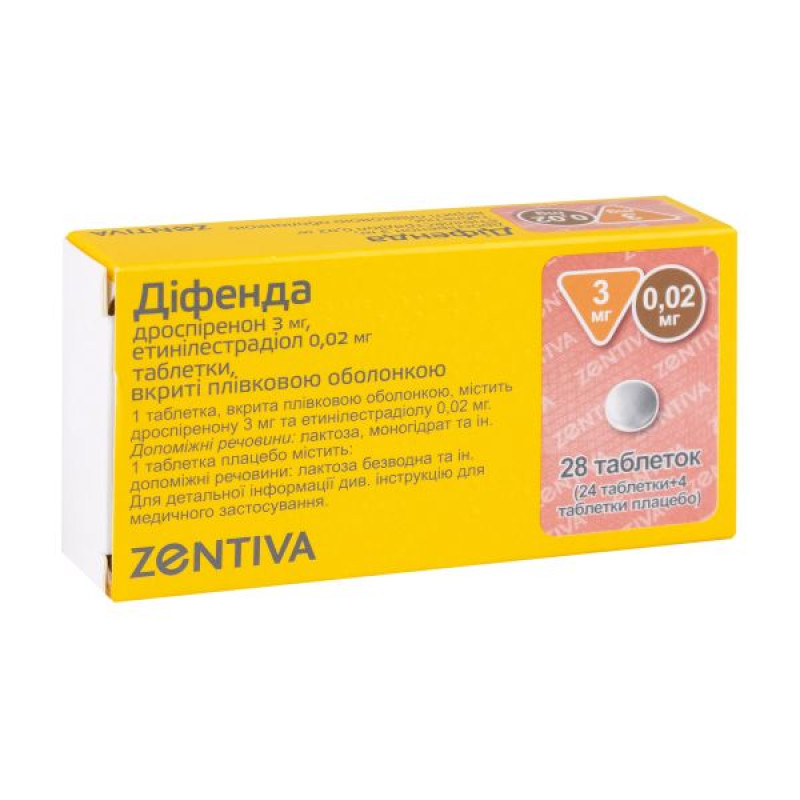
Instructions for use: Defense film-coated tablets 3 mg/0.02 mg blister (24+4) No. 28
Composition
active ingredients: drospirenone; ethinylestradiol;
1 pink tablet contains ethinylestradiol 0.02 mg and drospirenone 3 mg;
Excipients: lactose monohydrate; pregelatinized starch; povidone; croscarmellose sodium; polysorbate 80; magnesium stearate;
film coating: Opadry II Pink, containing: polyvinyl alcohol, titanium dioxide (E 171), macrogol, talc, iron oxide yellow (E 172), iron oxide red (E 172), iron oxide black (E 172);
1 white placebo tablet contains:
excipients: anhydrous lactose, povidone, magnesium stearate;
film coating: Opadry II white, containing: polyvinyl alcohol, titanium dioxide (E 171), macrogol, talc.
Dosage form
Film-coated tablets.
Main physicochemical properties: round, biconvex, pink film-coated tablets, and round, biconvex, white film-coated tablets (placebo).
Pharmacotherapeutic group
Sex hormones and drugs used in genital pathology. Hormonal contraceptives for systemic use. Progestogens and estrogens, fixed combinations. Drospirenone and ethinylestradiol. ATX code G0ZA A12.
Pharmacological properties
Pharmacodynamics.
Pearl Index of Contraceptive Failure: 0.41 (upper two-sided 95% confidence interval: 0.85).
Total Pearl Index (contraceptive failures + patient errors): 0.80 (upper two-sided 95% confidence interval: 1.30).
The contraceptive effect of the drug Difenda is based on the interaction of various factors, the most important of which are the suppression of ovulation and changes in cervical secretion.
In a three-cycle clinical trial of ovulation suppression comparing drospirenone 3 mg/ethinyl estradiol 0.02 mg in a 24-day regimen and a 21-day regimen, the 24-day regimen was associated with greater suppression of follicular development. After intentional dosing errors during the third cycle of therapy, the vast majority of women with the 21-day regimen
Ovarian activity, including ovulation, was observed compared to women on the 24-day regimen. Ovarian activity returned to pre-treatment levels within the post-therapy cycle in 91.8% of women on the 24-day regimen.
The drug Difenda is a combined oral contraceptive with ethinylestradiol and the progestogen drospirenone. In therapeutic doses, drospirenone exhibits antiandrogenic and moderate antimineralocorticoid properties. It has no estrogenic, glucocorticoid, or antiglucocorticoid activity. Therefore, drospirenone has a similar pharmacological profile to natural progesterone.
According to clinical studies, the moderate antimineralocorticoid properties of the drug Difenda lead to a moderate antimineralocorticoid effect.
Two multicenter, double-blind, randomized, placebo-controlled studies were conducted to evaluate the efficacy and safety of Defenda in women with moderate acne vulgaris. After 6 months of treatment, compared with placebo, the drug demonstrated a statistically significant reduction of 15.6% (49.3% vs. 33.7%) in inflammatory features, 18.5% (40.6% vs. 22.1%) in non-inflammatory features, and 16.5% (44.6% vs. 28.1%) in total lesions. A higher percentage of subjects, 11.8% (18.6% vs. 6.8%), had “clear” or “almost clear” skin as assessed by the Investigator's Stated Global Assessment (ISGA) scale.
Pharmacokinetics.
Drospirenone
Absorption. Orally administered drospirenone is rapidly and completely absorbed. The maximum serum concentration of 38 ng/ml is reached approximately 1-2 hours after a single oral dose. Bioavailability is 76-85%. Simultaneous food intake does not affect the bioavailability of drospirenone.
Distribution. After oral administration, the serum concentration of drospirenone decreases with a mean terminal half-life of about 31 hours. Drospirenone binds to serum albumin, but does not bind to sex steroids (SSBS) and corticoid-binding globulin (CBG). Only 3-5% of its total amount in serum is present in the free state. The increase in SSBS caused by ethinylestradiol does not affect the binding of drospirenone to serum proteins. The mean volume of distribution of drospirenone is 3.7±1.2 l/kg.
Elimination. The metabolic clearance rate of drospirenone from serum is approximately 1.5±0.2 ml/min/kg. Drospirenone is excreted unchanged only in very small amounts. Metabolites are excreted in urine and feces in a ratio of 1.2 to 1.4. The elimination half-life of metabolites in urine and feces is approximately 40 hours.
Steady state. During the application cycle, the maximum steady-state concentration of drospirenone in serum, which is approximately 70 ng/ml, is reached after 8 days of use. Drospirenone levels in the blood accumulated 3-fold as a result of the ratio of the terminal half-life and the dosing interval.
Certain groups of patients
Women with renal impairment. Steady-state serum concentrations of drospirenone in women with mild renal impairment (creatinine clearance 50-80 ml/min) were comparable to those in women with normal renal function (creatinine clearance >80 ml/min). Serum drospirenone levels were on average 37% higher in women with moderate renal impairment (creatinine clearance 30-50 ml/min) than in women with normal renal function. Drospirenone was well tolerated in all patient groups. Drospirenone has been shown to have no clinically significant effect on serum potassium.
Women with liver dysfunction.
In a single-dose study, oral clearance of drospirenone was reduced by approximately 50% in subjects with moderate hepatic impairment compared with volunteers with normal liver function. The observed difference in clearance of drospirenone in subjects with moderate hepatic impairment did not result in any apparent differences in serum potassium concentrations. Even in the presence of diabetes mellitus and concomitant therapy with spironolactone (two factors that can provoke hyperkalemia), no increase in serum potassium concentrations above the upper limit of normal was observed. Therefore, drospirenone is well tolerated in subjects with mild to moderate hepatic impairment (Child-Pugh class B).
Ethnic groups: No clinically significant differences in the pharmacokinetics of drospirenone or ethinyl estradiol were observed between Japanese and Caucasian women.
Ethinylestradiol
Absorption. Ethinylestradiol is rapidly and completely absorbed after oral administration. Peak serum concentrations of 33 pg/ml are reached within 1-2 hours after a single oral dose. Absolute bioavailability due to presystemic conjugation and first-pass metabolism is approximately 60%. Concomitant food intake reduces the bioavailability of ethinylestradiol in approximately 25% of subjects.
Distribution: Serum ethinylestradiol levels decline in a biphasic manner, with a terminal half-life of approximately 24 hours. Ethinylestradiol binds strongly but nonspecifically to serum albumin (approximately 98.5%) and induces an increase in serum concentrations of GH and CSH. The apparent volume of distribution is approximately 5 l/kg.
Metabolism: Ethinylestradiol is extensively metabolized in the gastrointestinal tract and during the first pass through the liver. Ethinylestradiol is metabolized mainly by hydroxylation of the aromatic ring to form a wide range of hydroxylated and methylated metabolites, which are present in the free state and as conjugates with glucuronides and sulfates. The metabolic clearance of ethinylestradiol is approximately 5 ml/min/kg.
In vitro, ethinylestradiol is a reversible inhibitor of CYP2C19, CYP1A1, and CYP1A2, and based on its mechanism of action, an inhibitor of CYP3A4/5, CYP2C8, and CYP2J2.
Excretion. Ethinylestradiol is almost not excreted unchanged. Ethinylestradiol metabolites are excreted in urine and bile in a ratio of 4:6. The half-life of metabolites is almost 1 day.
Steady state. Steady state is reached in the second half of the cycle of use, when the concentration of ethinylestradiol in the blood serum increases by 2.0-2.3 times.
Preclinical safety data.
In laboratory animals, the effects of drospirenone and ethinylestradiol were limited to those associated with the known pharmacological action. In particular, reproductive toxicity studies in animals showed species-specific embryotoxic and fetotoxic effects. At exposures exceeding those observed in users of the drug Difenda, effects on sexual differentiation were observed in some animal species. Ecological risk assessment studies showed that ethinylestradiol and drospirenone may potentially pose a threat to the aquatic environment (see section "Special precautions").
Indication
Oral contraception.
Contraindication
Combined hormonal contraceptives (CHCs) should not be used if any of the following conditions are present. If any of these conditions appear for the first time during CHC use, the drug should be discontinued immediately.
o Current VTE, particularly due to anticoagulant therapy, or history (e.g. deep vein thrombosis (DVT) or pulmonary embolism (PE));
o known hereditary or acquired predisposition for VTE, such as resistance to activated protein C (including factor V Leiden mutation), antithrombin-III deficiency, protein C deficiency, protein S deficiency;
o major surgical interventions with prolonged immobilization (see section "Peculiarities of use");
o high risk of VTE due to the presence of multiple risk factors (see section "Special warnings and precautions for use").
Presence or risk of arterial thromboembolism (ATE):
o current or history of ATE (e.g. myocardial infarction) or presence of prodromal symptoms (e.g. angina);
o current or history of cerebrovascular disease, presence of prodromal symptoms (e.g., transient ischemic attack (TIA));
o known hereditary or acquired predisposition to ATE, such as hyperhomocysteinemia and antiphospholipid antibodies (anticardiolipin antibodies, lupus anticoagulant);
o migraine with focal neurological symptoms in history;
o high risk of ATE due to the presence of multiple risk factors (see section "Special warnings and precautions for use") or due to the presence of one serious risk factor, such as:
diabetes mellitus with vascular complications;
severe arterial hypertension;
severe dyslipoproteinemia.
Current or history of severe liver disease until liver function tests return to normal.
Current or history of breast cancer, which may be hormone-sensitive (see section "Special warnings and precautions for use", subsection "Malignant neoplasms").
Severe renal failure or acute renal failure.
Presence of liver tumors at present or in history (benign or malignant).
Known or suspected malignant tumors (e.g., of the genital organs) that are sex hormone-dependent.
Vaginal bleeding of unknown etiology.
Hypersensitivity to the active substances or to any of the components of the drug.
Difenda is contraindicated for concomitant use with medicinal products containing ombitasvir/paritaprevir/ritonavir and dasabuvir, or medicinal products containing glecaprevir/pibrentasvir or sofosbuvir/velpatasvir/voxilaprevir (see section “Interaction with other medicinal products and other types of interactions”).
Special safety precautions
This medicinal product may pose a risk to the environment (see section 5.1). Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
Interaction with other medicinal products and other types of interactions
The information for the concomitant medicinal product should be consulted to identify potential interactions.
The effect of other medicines on the drug Difenda.
Interactions are possible with drugs that induce microsomal enzymes. This leads to an increase in the clearance of sex hormones, which causes changes in the pattern of menstrual bleeding and/or loss of contraceptive effectiveness.
Therapy
Enzyme induction may be detected after a few days of treatment. Maximum enzyme induction is generally observed after a few weeks. After discontinuation of treatment, enzyme induction may persist for about 4 weeks.
Short-term treatment
Women taking enzyme-inducing drugs should temporarily use a barrier method or another method of contraception in addition to the combined oral contraceptive (COC). The barrier method should be used throughout the duration of treatment with the drug and for 28 days after stopping its use. If therapy is started during the last COC tablet-free period, the next COC tablet-free period should be started immediately after the previous one without the usual tablet-free interval.
Long-term treatment
For women on long-term therapy with active substances that induce liver enzymes, a barrier or other appropriate non-hormonal method of contraception is recommended.
The following interactions have been reported based on published data.
Active substances that increase COC clearance (reduced COC efficacy due to enzyme induction), for example:
barbiturates, bosentan, carbamazepine, phenytoin, primidone, rifampicin; medicines used for HIV infection (ritonavir, nevirapine and efavirenz); also possibly felbamate, griseofulvin, oxcarbazepine, topiramate and herbal medicines containing St. John's wort extract (Hypericum perfiratum).
Active substances with inconsistent effects on COC clearance
Therefore, the prescribing information for the concomitant HIV/HCV medicinal product should be consulted for potential interactions and any other recommendations. In case of any doubt, women should additionally use a barrier method of contraception during treatment with protease inhibitors or non-nucleoside reverse transcriptase inhibitors.
Active substances that reduce COC clearance (enzyme inhibitors)
The clinical significance of the potential interaction with enzyme inhibitors remains unclear.
Concomitant use of strong CYP3A4 inhibitors may increase plasma concentrations of estrogen or progestin, or both.
In a multiple-dose study of the combination drospirenone (3 mg/day)/ethinyl estradiol
(0.002 mg/day) and the strong CYP3A4 inhibitor ketoconazole, administered concomitantly for 10 days, increased the AUC(0-24h) of drospirenone and ethinylestradiol in
2.7 and 1.4 times respectively.
Etoricoxib at doses of 60 to 120 mg/day has been shown to increase plasma concentrations of ethinylestradiol by 1.4- to 1.6-fold, respectively, when co-administered with a CHC containing 0.035 mg ethinylestradiol.
The effect of the drug Difenda on other drugs
COCs may affect the metabolism of some active substances. Accordingly, plasma and tissue concentrations may either increase (e.g. cyclosporine) or decrease (e.g. lamotrigine).
Based on in vivo interaction studies in female volunteers using omeprazole, simvastatin and midazolam as marker substrates, it was found that a clinically significant interaction of drospirenone at a dose of 3 mg with other active substances induced by cytochrome P450 is unlikely.
Clinical data suggest that ethinylestradiol inhibits the clearance of CYP1A2 substrates, causing a mild (e.g. theophylline) or moderate (e.g. tizanidine) increase in their plasma concentrations.
Pharmacodynamic interactions
In clinical trials in patients treated with hepatitis C virus (HCV) medicinal products containing ombitasvir/paritaprevir/ritonavir and dasabuvir with or without ribavirin, transaminase (ALT) elevations greater than 5 times the upper limit of normal (ULN) have been observed. This occurred at a significantly higher frequency in women taking ethinylestradiol-containing medicinal products, including combined hormonal contraceptives (CHCs). In addition, ALT elevations have been observed in women taking ethinylestradiol-containing medicinal products, such as CHCs, in patients treated with glecaprevir/pibrentasvir or sofosbuvir/velpatasvir/voxilaprevir (see section 4.3).
Therefore, patients should switch to an alternative method of contraception (e.g. progestogen-only contraception or non-hormonal methods) before starting this combination regimen. Use of Difend may be resumed 2 weeks after completion of the combination regimen.
In patients with normal renal function, concomitant use of drospirenone and ACE inhibitors or nonsteroidal anti-inflammatory drugs (NSAIDs) has not shown a significant effect on
Serum potassium levels. However, concomitant use of Defenda with aldosterone antagonists or potassium-sparing diuretics has not been studied. In this case, serum potassium levels should be monitored during the first treatment cycle (see also section 4.4).
Other forms of interaction
Laboratory tests
The use of contraceptive steroids may affect the results of some laboratory tests, such as biochemical parameters of liver, thyroid, adrenal and renal function, plasma concentrations of transport proteins such as corticosteroid-binding globulin, plasma concentrations of lipid/lipoprotein fractions, and parameters of carbohydrate metabolism, coagulation and fibrinolysis. These changes are usually within normal limits. Drospirenone increases plasma renin and aldosterone activity, which is induced by its moderate antimineralocorticoid activity.
Application features
The decision to prescribe Defenda should take into account the woman's individual current risk factors, including risk factors for VTE, and the risk of VTE associated with Defenda compared with other CHCs (see sections 4.3 and 4.4).
Warning
If any of the conditions or risk factors listed below are present, the appropriateness of using Difenda should be discussed with the woman.
In the event of exacerbation or at the first manifestations of any of the above conditions or risk factors, women are advised to consult a doctor and determine the need to discontinue taking Difenda.
In case of suspected or confirmed VTE or ATE, CHC use should be discontinued. If anticoagulant therapy is initiated, alternative adequate contraception should be provided due to the teratogenic effects of anticoagulants (coumarins).
Circulatory disorders
The use of any CHC increases the risk of VTE in women who use it compared with those who do not use it. Products containing levonorgestrel, norgestimate or norethisterone are associated with a lower risk of VTE. The use of other products such as Difenda may lead to a twofold increase in risk. The decision to use products other than those with the lowest risk of VTE should only be made after discussion with the woman. It should be ensured that she is aware of the risk of VTE associated with Difenda, the extent of her existing risk factors and the fact that the risk of VTE is highest during the first year of use. Some data suggest that the risk of VTE may increase when CHC use is resumed after a break of 4 weeks or more.
Two in 10,000 women who are not taking CHCs and are not pregnant will develop a VTE in a year. However, the risk for an individual woman may be much higher depending on the risk factors she has (see below).
It has been estimated1 that out of 10,000 women using a CHC containing drospirenone, 9–12 will develop a VTE in one year. This compares with 62 in women using a CHC containing levonorgestrel.
In both cases, the number of VTE cases per year was lower than would normally be expected during pregnancy or in the postpartum period.
VTE can be fatal in 1-2% of cases.
Number of VTE cases per 10,000 women in one year
1 These figures are based on all epidemiological data, taking into account the relative risks associated with the use of different CHCs compared with the use of CHCs containing levonorgestrel.
2 On average 5-7 cases per 10,000 women-years based on the calculation of the relative risk of using levonorgestrel-containing CHCs compared to that in women not using CHCs (approximately 2.3-3.6 cases).
Risk factors for developing VTE
The risk of developing venous thromboembolic complications in women using CHCs may be significantly higher in the presence of additional risk factors, especially multiple ones (see Table 2).
The use of Difenda is contraindicated in women with multiple risk factors that may increase the risk of venous thrombosis (see section "Contraindications"). If a woman has more than one risk factor, the increased risk may be greater than the sum of the risks associated with each individual factor, so the overall risk of VTE should be considered. If the benefit/risk ratio is unfavorable, CHCs should not be prescribed (see section "Contraindications").
Table 2
Risk factors for developing VTE
| Risk factor | Note |
| Obesity (body mass index greater than 30 kg/m2) | The risk increases significantly with increasing body mass index. It especially requires attention in the presence of other risk factors. |
Prolonged immobilization, major surgery, surgery on the lower extremities or pelvic organs, neurosurgery, or extensive trauma. Note: Temporary immobilization, including flights > 4 hours, may also be a risk factor for VTE, especially in women with other risk factors. | In such situations, it is recommended to discontinue use of the drug (in the case of elective surgery at least 4 weeks in advance) and not resume use earlier than 2 weeks after full recovery of motor activity. In order to avoid unwanted pregnancy, other methods of contraception should be used. Antithrombotic therapy should be considered if Defenda has not been discontinued beforehand. |
| Family history (VTE in a relative or parent, especially at a relatively young age, such as under 50 years of age). | In case of hereditary predisposition, women are advised to consult a specialist before using any CHC. |
| Other conditions associated with VTE | Cancer, systemic lupus erythematosus, hemolytic uremic syndrome, chronic inflammatory bowel disease (Crohn's disease or ulcerative colitis), and sickle cell anemia. |
| Age | Especially over the age of 35 |
There is no consensus on the possible impact of varicose veins and superficial thrombophlebitis on the development and progression of venous thrombosis.
Attention should be paid to the increased risk of thromboembolism during pregnancy, especially during the 6 weeks after delivery (for information on pregnancy or breastfeeding, see section "Use during pregnancy or breastfeeding").
Symptoms of VTE (deep vein thrombosis (DVT) and pulmonary embolism (PE))
Women should be advised to contact their doctor immediately if they experience the following symptoms and to inform them that they are using CHCs.
Symptoms of DVT may include: unilateral swelling of the leg and/or foot or areas along a vein in the leg; pain or increased sensitivity in the leg that may only be felt when standing or walking; a feeling of heat in the affected leg; redness or discoloration of the skin on the leg.
Some of these symptoms (e.g., shortness of breath, cough) are nonspecific or may be misinterpreted as more common or less severe conditions (e.g., respiratory tract infections).
Other manifestations of vascular occlusion may include sudden pain, swelling, acute abdomen, and slight blueness of the limb.
In the case of occlusion of the vessels of the eye, the initial symptom may be blurred vision, which is not accompanied by pain, and which can progress to loss of vision. Sometimes the loss of vision develops almost instantly.
Risk of developing ATE
Epidemiological studies have shown that the use of any CHC is associated with an increased risk of ATE (myocardial infarction) or cerebrovascular events (TIA, stroke). Arterial thromboembolic events can be fatal.
Risk factors for developing ATE
The risk of arterial thromboembolic complications or cerebrovascular events is increased in women with risk factors when using CHCs (see Table 3). The use of the drug Defenda is contraindicated if women have one serious or multiple risk factors for ATE that may increase the risk of developing arterial thrombosis (see section "Contraindications"). If a woman has more than one risk factor, the increased risk may be greater than the sum of the risks associated with each individual factor, so the overall risk should be considered. If the benefit/risk ratio is unfavorable, CHCs should not be prescribed (see section "Contraindications").
Table 3
Risk factors for developing ATE
| Risk factor | Note |
| Increasing age | Especially over the age of 35 |
| Smoking | Women using CHCs are advised to abstain from smoking. Women over 35 who continue to smoke are strongly advised to use another method of contraception. |
| Arterial hypertension | |
| Obesity (body mass index greater than 30 kg/m2) | The risk increases significantly with increasing body mass index. It is especially important to pay attention to women who have other risk factors. |
| Family history (ATE in a relative or parent, especially at a relatively young age, such as under 50 years of age) | In case of hereditary predisposition, women are advised to consult a specialist before using any CHC. |
| Migraine | An increase in the frequency or severity of migraine during CHC use (possible prodromal states before the development of cerebrovascular events) may be a reason for immediate discontinuation of CHC use. |
| Other conditions associated with adverse vascular reactions | Diabetes mellitus, hyperhomocysteinemia, heart valve defects, atrial fibrillation, dyslipoproteinemia, and systemic lupus erythematosus. |
Symptoms of ATE
Women should be advised to contact their doctor immediately if they experience the following symptoms and to inform them that they are using CHCs.
Symptoms of a cerebrovascular accident may include: sudden numbness of the face, weakness or numbness of the limbs, especially on one side; sudden trouble walking, dizziness, loss of balance or coordination; sudden confusion, trouble speaking or understanding; sudden loss of vision in one or both eyes; sudden, severe or prolonged headache with no known cause; loss of consciousness or fainting with or without seizures.
The transient nature of symptoms may indicate a TIA.
Symptoms of a myocardial infarction may include: pain, discomfort, a feeling of squeezing or heaviness in the chest, arm or below the breastbone; discomfort radiating to the back, jaw, throat, arm, stomach; a feeling of fullness in the stomach, indigestion or shortness of breath; increased sweating, nausea, vomiting or dizziness; extreme weakness, anxiety or shortness of breath; fast or irregular heartbeat.
Malignant neoplasms
The results of some epidemiological studies indicate an additional increase in the risk of developing cervical cancer with long-term use of COCs (> 5 years), but this statement remains controversial, since it is not yet clear to what extent the results of the studies take into account concomitant risk factors, such as sexual behavior, and other factors, such as human papillomavirus infection.
In isolated cases, benign and even more rarely malignant liver tumors have been observed in women using COCs, which in some cases led to life-threatening intra-abdominal bleeding. In case of complaints of severe epigastric pain, liver enlargement or signs of intra-abdominal bleeding, the possibility of a liver tumor should be considered in the differential diagnosis when using COCs.
High-dose COCs (50 mcg ethinylestradiol) reduce the risk of endometrial and ovarian cancer. It remains to be confirmed whether these findings can also be extended to low-dose COCs.
Breast cancer (warnings issued by the FDA's Center for Drug Evaluation and Research (CDER))
Drospirenone/ethinylestradiol is contraindicated in women with current or history of breast cancer, as breast cancer may be hormone-sensitive (see section 4.3).
Epidemiological studies have not found a consistent association between the use of combined oral contraceptives (COCs) and the risk of breast cancer. Studies have not shown an association between current or past use of COCs and the risk of breast cancer. However, some studies have reported a small increased risk of breast cancer among current or recent users of COCs (< 6 months since last use) compared with never users of COCs (see section 4.8).
Other states
The progestin component of Difenda is an aldosterone antagonist with potassium-sparing properties. In most cases, an increase in potassium levels is not expected. In clinical studies, in some patients with mild to moderate renal insufficiency and concomitant use of potassium-sparing drugs, serum potassium levels increased slightly, but not significantly, during the use of drospirenone. Therefore, monitoring of potassium levels is recommended during the first cycle of treatment in patients with renal insufficiency. These patients are also recommended to maintain serum potassium levels not above the upper limit of normal before starting the drug, especially when concomitantly using potassium-sparing drugs (see section “Interaction with other medicinal products and other forms of interaction”).
Women with hypertriglyceridemia or a family history of this disorder are at risk of developing pancreatitis when using COCs.
Although a slight increase in blood pressure has been reported in many women taking COCs, clinically significant increases in blood pressure have been observed in isolated cases. Immediate discontinuation of COCs is only necessary in these isolated cases. In the case of persistent hypertension or failure to control blood pressure with antihypertensive drugs, women taking COCs should discontinue their use. If necessary, COC use can be resumed after achieving normotensive status with antihypertensive therapy.
The following conditions have been reported to occur or worsen during pregnancy and COC use, but the relationship to estrogen/progestin use is not conclusive: jaundice and/or pruritus associated with cholestasis, gallstone formation, porphyria, systemic lupus erythematosus, hemolytic uremic syndrome, Sydenham's chorea, herpes gestationis, hearing loss associated with otosclerosis.
Exogenous estrogens may induce or exacerbate symptoms of hereditary and acquired angioedema.
Acute or chronic liver function disorders may require discontinuation of COC use until liver function tests return to normal and a causal relationship to COC use has been ruled out.
If cholestatic jaundice and/or itching associated with cholestasis recurs, which previously occurred during pregnancy or previous use of sex hormones, COC use should be discontinued.
Although
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.