Divigel gel 0.1% sachet 1 g No. 28

Instructions Divigel gel 0.1% sachet 1 g No. 28
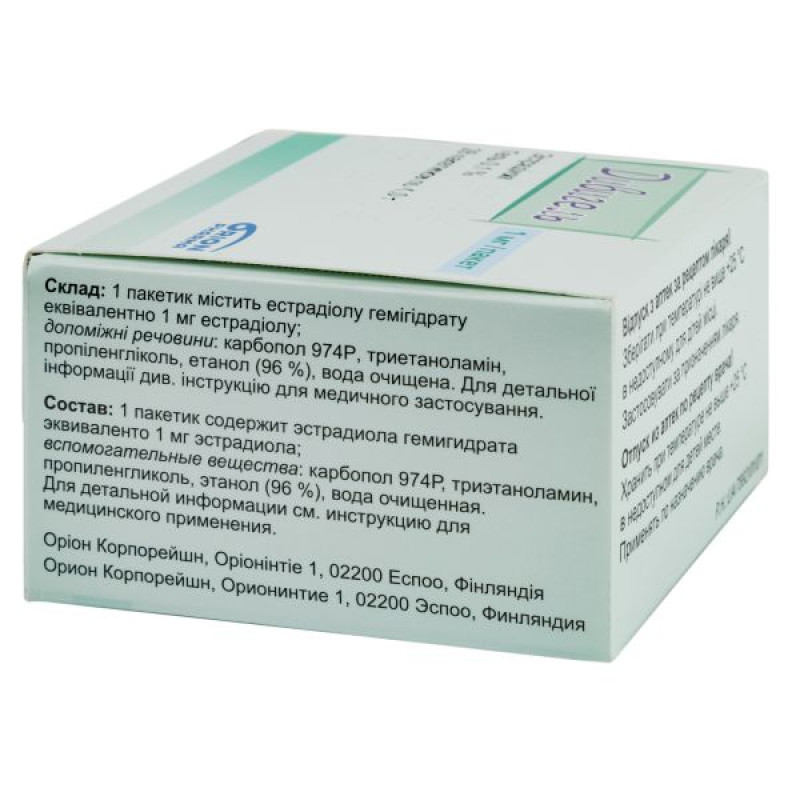
Composition
active ingredient: estradiol;
1 sachet contains estradiol hemihydrate equivalent to 0.5 mg or 1 mg of estradiol;
excipients: carbomer 974P, triethanolamine, propylene glycol, ethanol 96%, purified water.
Dosage form
Gel.
Main physicochemical properties: homogeneous opalescent gel.
Pharmacotherapeutic group
Simple preparations of natural and semi-synthetic estrogens. Estradiol. ATX code G03C A03.
Pharmacological properties
Pharmacodynamics
Estradiol valerate, which is a synthetic 17b-estradiol, is chemically and biologically identical to endogenous human estradiol. It compensates for the decreased estrogen levels in menopausal women, thereby alleviating menopausal symptoms. Estrogens prevent bone loss that occurs during menopause or after oophorectomy.
Transdermal application of estradiol in combination with medroxyprogesterone acetate helps to reduce total cholesterol levels without changing high-density lipoprotein cholesterol levels. The effectiveness of Divigel for correcting postmenopausal bone loss is the same as with oral estrogens.
Pharmacokinetics
Divigel is an alcohol-based estradiol gel.
When the gel is applied to the skin, the alcohol evaporates quickly and estradiol penetrates through the skin into the circulatory system. Applying the drug to an area of 200-400 cm2 (the size of one or two palms) does not affect the amount of estradiol absorbed. However, if the drug is applied to a large area, the degree of absorption is significantly reduced. Some estradiol is retained in the subcutaneous tissue, from where it gradually penetrates into the systemic bloodstream. Transdermal application avoids the first stage of hepatic metabolism. For this reason, fluctuations in the concentration of estrogen in the blood plasma when using Divigel are much less pronounced than when using oral estrogens.
With transdermal application of Divigel, plasma estradiol concentrations were as follows:
| Divigel dose | Cmax (pmol/L) | Average (pmol/l) | Cmin(pmol/l) |
| 0.5 mg | 143 | 75 | 92 |
| 1 mg | 247 | 124 | 101 |
| 1.5 mg | 582 | 210 | 152 |
During treatment with the drug, the estradiol/estrone ratio is maintained at 0.4-0.7, whereas with oral estrogens it usually falls to less than 0.2. The steady-state bioavailability is 82% compared to an equivalent oral dose of estradiol valerate.
The metabolism and excretion of Divigel when used transdermally are similar to those of natural estrogens.
Indication
Symptoms associated with estrogen deficiency, during natural or artificial menopause.
For the prevention of postmenopausal osteoporosis with a high risk of fracture when other medicines for the prevention of osteoporosis are contraindicated or unsuitable.
Contraindication
Breast cancer (diagnosed, suspected or history).
Known or suspected estrogen-dependent malignant tumors (e.g. endometrial cancer).
Vaginal bleeding of unknown etiology.
Untreated endometrial hyperplasia.
Thromboembolic venous diseases, including a history (deep vein thrombosis (DVT), pulmonary embolism).
Increased blood clotting has been detected (for example, protein C, protein S, or antithrombin deficiency).
Acute arterial thromboembolism, including a history (e.g. angina pectoris, myocardial infarction).
Acute liver diseases, including a history (until normalization of laboratory liver function tests).
Hypersensitivity to the active substance or to any of the excipients.
Porphyria.
Interaction with other medicinal products and other types of interactions
Concomitant use of drugs that are inducers of hepatic enzymes, particularly cytochrome P450, may accelerate the metabolism of estradiol. These substances include anticonvulsants (e.g., phenobarbital, phenytoin, carbamazepine) and antimicrobials (e.g., rifampicin, rifabutin, nevirapine, efavirenz).
Ritonavir and nelfinavir, although they are potent inhibitors, when used simultaneously with steroid hormones, they exhibit, on the contrary, an inducing effect.
Herbal preparations containing St. John's wort may increase estrogen metabolism. A clinically significant increase in estrogen metabolism may result in decreased effectiveness of these hormones and may cause changes in menstrual bleeding patterns.
Clinically increased metabolism of estrogens and progestogens may lead to a decrease in the effect and a change in the pattern of vaginal bleeding.
Application features
There is limited experience with hormone replacement therapy (HRT) in the treatment of women aged 65 and over.
HRT should only be used if you have postmenopausal symptoms that negatively affect your quality of life.
The risk-benefit ratio of therapy should be carefully assessed (at least annually).
Information on side effects associated with hormone replacement therapy for premature menopause is limited. Because of the low absolute risk of adverse reactions in younger women, the risk-benefit ratio for these women may be more favorable than for older women.
Examination.
Before starting or re-prescribing hormone replacement therapy (HRT), the doctor should collect a complete personal and family history of the patient, conduct a medical examination (including pelvic organs and mammary glands) to identify possible contraindications and exercise due caution when prescribing the drug.
Periodic examinations are recommended during treatment. The frequency and type of examination methods are determined for each patient individually. Women should be informed about what changes in the mammary glands should be reported to the doctor. Examinations, including mammography, should be performed in accordance with accepted standards and adapted to the individual clinical needs of each patient.
Conditions that require control.
If any of the following conditions are present, have occurred previously and/or have been aggravated during pregnancy or previous hormonal therapy, the patient should be under constant medical supervision. These conditions may in some cases recur or be aggravated during treatment with Divigel: uterine leiomyoma (fibroid) or endometriosis, thromboembolic diseases, if they were in the past or their threat, risk factors for estrogen-dependent tumors (for example, I degree of heredity of breast cancer), arterial hypertension, liver disease (for example, liver adenoma), diabetes mellitus with or without vascular damage, cholelithiasis, migraine or severe headache, systemic lupus erythematosus, a history of endometrial hyperplasia, epilepsy, asthma, otosclerosis, hereditary angioedema.
Reasons for immediate discontinuation of therapy.
Therapy should be discontinued if contraindications are identified and if the following conditions occur: jaundice or deterioration of liver function, marked increase in blood pressure, new attacks of migraine-like headache, pregnancy.
Endometrial hyperplasia and carcinoma.
The risk of endometrial hyperplasia and cancer increases when patients with an intact uterus are treated with estrogen alone for long periods of time. A 2- to 12-fold increased risk of endometrial cancer has been reported, depending on the duration of treatment and the dose of estrogen, in patients who used estrogens compared with those who did not.
After stopping treatment, the risk may remain elevated for at least 10 years.
In women with an intact uterus, continuous combined estrogen and progestogen therapy or cyclical addition of a progestogen, for at least 12 days per month or for 28 days, prevents the risk associated with increased estrogen.
Breakthrough bleeding and spotting may occur during the first month of treatment. If bleeding or spotting occurs some time after starting treatment or if it continues after stopping treatment, the cause of the bleeding should be investigated. If necessary, an endometrial biopsy should be performed to exclude malignant transformation of the cells.
Estrogen therapy without the addition of a progestogen may lead to precancerous or malignant changes in the endometriotic foci. Therefore, the advisability of adding a progestogen to estrogen therapy should be considered for patients who have undergone hysterectomy for endometriosis, especially for patients with identified foci of endometriosis.
Breast cancer.
The risk of developing breast cancer is increased both in patients who use a combination of estrogen and progestogen, and in those who use estrogen alone.
Estrogen-progestogen combination therapy.
Randomized, placebo-controlled, and epidemiological studies have shown that the use of combined estrogen and progestogen for hormone replacement therapy increases the risk of breast cancer. The effect becomes apparent after about 3 years.
Estrogen monotherapy.
In women who have had a hysterectomy, the use of estrogens for hormone replacement therapy does not increase the risk of breast cancer.
Other observations show a small increased risk of breast cancer, but this increase is much smaller than with the use of estrogen-progestogen combinations (see section "Adverse reactions").
There is a clear increase in the risk of cancer with several years of estrogen therapy. However, the risk returns to baseline within a few (no more than five) years after stopping treatment.
HRT, especially estrogen-progestogen combinations, increase breast tissue density, which may lead to a deterioration in the radiological diagnosis of breast cancer.
Ovarian cancer.
Ovarian cancer is much less common than breast cancer. Long-term use (at least 5 to 10 years) of estrogen-only HRT has been associated with a small increased risk of ovarian cancer. Some studies suggest that long-term use of combined HRT may be associated with a similar or even lower risk (see section 4.8).
HRT is associated with a 1.3- to 3-fold increased risk of developing venous thromboembolism, such as deep vein thrombosis or pulmonary embolism. The development of the disease is most likely in the first year of hormone replacement therapy (see section "Adverse reactions").
Patients with a predisposition to thrombosis have a significant risk of developing venous thromboembolism. Since HRT may increase this risk, it is contraindicated in such patients (see section 4.3).
Common risk factors for venous thromboembolism include estrogen use, older age, major surgery, prolonged immobilization, obesity (BMI > 30 kg/m2), pregnancy/postpartum period, systemic lupus erythematosus, and cancer. There is no consensus on the possible role of varicose veins in the development of venous thromboembolism.
For all surgical patients, the need for special prophylactic measures to prevent postoperative thromboembolism should be considered. If the patient needs to be immobilized for a long time after elective surgery, it is recommended to stop hormone replacement therapy 4 to 6 weeks before surgery. Treatment should not be resumed until adequate motor activity has been restored.
If a woman's first-degree relatives have had venous thromboembolism at a young age, screening for thrombophilia should be considered. The woman should be informed beforehand that the screening has limited information (it only identifies some of the disorders that can lead to thrombophilia). If an increased tendency to thrombus formation is established, there are known cases of thrombosis in relatives, or the established disorder is serious (for example, deficiency of antithrombin, protein S and/or C, or a combination of these conditions), HRT is contraindicated.
If the patient is taking anticoagulants for a long time, the benefits and risks of hormone replacement therapy should be carefully weighed.
Treatment should be discontinued if venous thromboembolism develops early in therapy. The patient should be informed of the symptoms of thromboembolism that should prompt immediate medical attention (e.g., painful swelling of the leg, sudden chest pain, difficulty breathing).
Ischemic heart disease.
Randomized controlled trials have not shown a protective effect of combined estrogen-progestin hormone replacement therapy or estrogen monotherapy in women with coronary heart disease or in other women.
Estrogen-progestogen combination therapy.
The relative risk of developing coronary heart disease is slightly increased with combined estrogen-progestagen therapy. The absolute risk of developing coronary heart disease depends on age. The number of cases of coronary heart disease as a result of combined estrogen-progestagen therapy in healthy women close to the menopause is very small, but increases with age.
Estrogen monotherapy.
Randomized controlled trials have not found an increased risk of coronary heart disease in women after hysterectomy who used estrogen alone.
Ischemic stroke.
Estrogen-progestogen combination therapy and estrogen monotherapy are associated with a 1.5-fold increased risk of ischemic stroke. The relative risk does not change with age or after menopause. Since the risk of stroke is strongly age-dependent, the overall risk of stroke in women taking HRT increases with age (see section 4.8).
Other features.
Estrogens can cause fluid retention in the body, so patients with impaired heart and kidney function should be under special supervision.
Patients with hypertriglyceridemia who are taking HRT should be carefully monitored. There have been several cases of a sharp increase in plasma triglyceride levels when estrogens were used in such patients, which may lead to the development of pancreatitis.
Estrogens increase thyroid-binding globulin (TBG), which leads to increased circulating thyroid hormone levels as measured by protein-bound iodine, T4 concentration (column or radioimmunoassay), or T3 concentration (radioimmunoassay). Increased T3 levels are reduced, reflecting increased TBG levels, and free T4 and T3 concentrations are altered.
Serum concentrations of other binding proteins, such as corticoid-binding globulin (CBG), sex hormone-binding globulin (SHBG), may be increased, leading to increased concentrations of circulating corticosteroids and sex steroid hormones, respectively. Free or biologically active hormone concentrations remain unchanged. Other plasma proteins may be increased (angiotensin/renin substrate, alpha-1-trypsin, ceruloplasmin).
Chloasma may occasionally occur, especially in women with a history of chloasma during pregnancy. Women with a predisposition to chloasma should minimize exposure to sunlight or ultraviolet radiation during HRT.
HRT does not improve cognitive function. There is some evidence that the risk of dementia increases in women aged 65 years and older at the start and during estrogen therapy.
Divigel contains propylene glycol, which may irritate the skin.
Potential exposure of estradiol to the child
Gel containing estradiol can accidentally enter the child's body through contact with the area of skin to which it was applied.
In the post-marketing period, cases of breast enlargement in prepubertal girls, precocious puberty and gynecomastia in prepubertal boys have been reported following inadvertent secondary exposure to estradiol-containing gel.
In most cases, the condition normalized after cessation of estradiol exposure.
Patients should be instructed about the need to:
- prevent contact of other people, especially children, with exposed skin and, if necessary, cover the application site with clothing. In case of contact of the application site with the child's skin, it is necessary to wash it with soap and water as soon as possible;
- consult a doctor if a child who may have been accidentally exposed to gel containing estradiol develops signs and symptoms such as breast enlargement or other sexual changes.
Use during pregnancy or breastfeeding
Divigel is not indicated for use during pregnancy or breastfeeding. If the patient becomes pregnant during therapy, Divigel treatment should be discontinued immediately.
According to the results of most epidemiological studies, accidental use of estrogen during pregnancy does not have a teratogenic or fetotoxic effect.
Ability to influence reaction speed when driving vehicles or other mechanisms
The drug does not affect the reaction speed when driving vehicles or other mechanisms.
Method of administration and doses
Dosage.
Divigel is a transdermal gel intended for long-term or cyclical treatment. The usual initial dose is 1 g of gel per day, which corresponds to 1 mg of estradiol. The duration of use and dose are selected by the doctor taking into account the individual characteristics of the patient (depending on the clinical condition, after 2–3 cycles the dose can be adjusted: from 0.5 g to 1.5 g of gel per day, which corresponds to 0.5–1.5 mg of estradiol per day).
For patients with an intact uterus, Divigel should be combined with progestogen therapy at 1-month intervals, using, for example, medroxyprogesterone acetate, norethindrone, norethindrone acetate or dydrogesterone for at least 12–14 days.
Progestogens are not recommended for women who have had a hysterectomy unless they have been diagnosed with endometriosis.
Patients who have not previously used hormone replacement therapy (HRT) or who are switching to Divigel after long-term combined therapy can start treatment with Divigel on any day. Patients who are switching to Divigel from continuous hormone replacement therapy can start treatment with Divigel after the end of the last treatment cycle.
When treating postmenopausal symptoms, the lowest effective dose should be used, and the duration of treatment should be as short as possible.
If the patient forgets to apply the gel on time, treatment should be continued as usual the next day.
With irregular use of the drug, menstrual-like uterine bleeding may occur.
Method of application.
Divigel should be applied to clean, dry skin.
Apply a dose of Divigel once a day to the skin of the thighs or lower body, regularly changing the application site. The application area is 1–2 palms in size. Divigel should not be applied to the mammary glands, face, genitals, or irritated skin areas. After applying the drug, wait a few minutes for the gel to dry. The application site should not be washed for one hour. Accidental contact of Divigel with the eyes should be avoided. Hands should be washed immediately after applying the gel.
Patients should be advised that children should not come into contact with the area of the body where estradiol-containing gel has been applied (see section 4.4).
Children
Do not use the drug in children.
Overdose
Acute toxicity studies do not indicate a risk of acute adverse reactions after administration of a dose several times higher than the recommended dose.
Some women may experience nausea, headache, vomiting, and withdrawal bleeding.
According to several reports, no serious side effects have been observed in children who took oral contraceptives with a high dose of estrogen.
Treatment is symptomatic.
Overdose of estradiol is unlikely when applied transdermally. There is no specific antidote. Treatment is symptomatic. The gel should be washed off.
Adverse reactions
During the first few months of treatment, breakthrough bleeding and spotting, breast tenderness or enlargement may occur. These symptoms are usually short-lived and disappear during treatment.
The data presented below are from clinical trials and post-marketing experience. Adverse reactions may occur in approximately 76% of patients. Adverse reactions reported in more than 10% of patients in clinical trials were application site reactions and breast pain.
Adverse reactions that occurred with transdermal use of estradiol.
Benign, malignant and unspecified neoplasms (including cysts and polyps).
Uncommon: benign breast and endometrial tumors.
Frequency unknown: myoma.
Immune system disorders.
Uncommon: hypersensitivity reactions.
Frequency unknown: exacerbation of hereditary angioedema.
Metabolic and nutritional disorders.
Common: edema, weight gain, weight loss.
Uncommon: increased appetite.
Frequency not known: hypercholesterolemia1.
Mental disorders.
Common: depression, nervousness, drowsiness.
Uncommon: changes in libido and mood, anxiety, insomnia, apathy, emotional lability, impaired concentration.
Frequency unknown: euphoria1, excitement1.
Nervous system disorders.
Common: headache, dizziness.
Uncommon: migraine, paresthesia.
Frequency unknown: tremor1.
Visual impairment.
Uncommon: visual disturbances.
Rare: contact lens intolerance.
Frequency unknown: dry eyes1.
Cardiovascular system disorders.
Often: hot flashes.
Uncommon: palpitations.
Rare: increased blood pressure, venous thromboembolism (e.g. deep vein thrombosis of the lower extremities or pelvic vein thrombosis), pulmonary embolism2.
Frequency unknown: cerebrovascular accident, superficial phlebitis1, purpura1.
Respiratory system disorders, thoracic and mediastinal disorders.
Frequency not known: difficulty breathing1, rhinitis1.
Digestive tract disorders.
Common: nausea, vomiting, gastric colic, flatulence.
Uncommon: constipation.
Frequency unknown: abdominal pain, bloating, dyspepsia1, diarrhoea1, rectal symptoms1.
Hepatobiliary system disorders.
Rare: impaired liver function and bile flow.
Frequency unknown: cholestatic jaundice.
Skin and subcutaneous tissue disorders.
Uncommon: acne, alopecia, dry skin, erythema nodosum, urticaria.
Rare: rash.
Frequency unknown: contact dermatitis, eczema, nail plate changes1, nodular skin changes1, hirsutism1.
Disorders of the musculoskeletal system and connective tissue.
Uncommon: joint symptoms, muscle cramps.
Kidney and urinary system disorders.
Uncommon: increased frequency of urges and urination.
Frequency not known: urinary incontinence1, cystitis1, urine discoloration1, haematuria1.
Disorders of the reproductive system and mammary glands.
Common: breast tenderness/pain/tenderness, breakthrough bleeding or spotting, vaginal discharge, vulvar/vaginal symptoms, menstrual disorders.
Uncommon: breast enlargement, breast tenderness, endometrial hyperplasia.
Rare: painful menstruation, premenstrual syndrome.
Frequency unknown: uterine symptoms1.
General disorders and administration site reactions.
Common: irritation, itching at the application site, pain, hyperhidrosis.
Uncommon: fatigue.
Frequency unknown: laboratory abnormalities1, asthenia1, hyperthermia1, influenza-like symptoms1, malaise1.
1 Isolated reports. Due to the small number of subjects in the studies (n = 611), the adverse reactions listed cannot be classified as uncommon or rare based on the results obtained.
2 See sections “Contraindications” and “Special instructions for use”.
Other adverse reactions that have occurred with estrogen-progestogen combination therapy:
Estrogen-dependent benign and malignant tumors, such as endometrial cancer.
Development of myocardial infarction and stroke.
Gallbladder dysfunction.
Isolated cases of chloasma, erythema multiforme.
Development of dementia after the age of 65 (see section "Special warnings and precautions for use").
Risk of developing breast cancer.
The risk of developing breast cancer is increased in women who have used a combination of estrogen and progestogen for more than five years. The risk is much lower in women who have used estrogen monotherapy than in those who have used an estrogen-progestogen combination. The degree of risk depends on the duration of treatment (see section "Special instructions").
Risk of developing endometrial cancer.
Postmenopausal patients with an intact uterus.
The risk of developing endometrial cancer in women with an intact uterus who do not use HRT is 5:1000.
Estrogen monotherapy is not recommended for HRT in women with an intact uterus, as the risk of endometrial cancer is increased (see section "Special warnings and precautions for use").
According to epidemiological studies, the increased risk of endometrial cancer depends on the duration of estrogen monotherapy and the dose of estrogen and ranges from 5 to 55 additional cases per 1,000 women aged 50 to 65 years.
Adding progestogens to estrogen therapy for at least 12 days during each period prevents this increase in risk.
According to the MWS (Million Women Study), the use of combined HRT (cyclic or continuous) for 5 years does not increase the risk of developing endometrial cancer [(relative risk 1.0 (95% CI 0.8–1.2))].
Risk of developing ovarian cancer.
According to studies, over 5 years of HRT in women aged 50 to 54, approximately 1 case of ovarian cancer was recorded per 2,000 patients who received HRT and 2 cases of ovarian cancer per 2,000 patients who did not receive HRT.
Risk of developing venous embolism.
HRT is associated with a 1.3- to 3-fold increased risk of developing venous thromboembolism, such as deep vein thrombosis or pulmonary embolism. The development of the disease is most likely in the first year of HRT.
Risk of developing coronary heart disease.
The risk of developing coronary heart disease is slightly increased in patients aged 60 years and older who use HRT.
Risk of developing ischemic stroke.
The relative risk of ischemic stroke increases 1.5-fold in patients using both estrogen monotherapy and estrogen-progestogen combination therapy. The risk of hemorrhagic stroke is not increased during HRT.
The relative risk does not depend on age or duration of treatment, but the risk itself is clearly age-dependent, so the overall risk of stroke increases with age in patients using HRT (see section "Special warnings and precautions for use").
Expiration date
3 years.
Storage conditions
Store at a temperature not exceeding + 25 °C in a place inaccessible to children.
Packaging
0.5 g or 1 g of gel in a sachet; 28 sachets in a cardboard box.
Vacation category
According to the recipe.
Producer
Orion Corporation.
Location of the manufacturer and its business address
Orionintie 1, 02200 Espoo, Finland.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.