Dr. Mom ointment jar of 20 g

Instructions for Dr. Mom ointment jar 20 g
Composition
active ingredients: 1 g of ointment contains menthol (levomenthol) 30.5 mg, camphor 52.5 mg, thymol 1 mg, turpentine oil 0.056 ml, eucalyptus oil 0.015 ml, nutmeg oil 0.005 ml;
excipients: white soft paraffin.
Dosage form
Ointment.
Main physicochemical properties: translucent ointment from white to almost white in color with a characteristic odor of menthol and camphor.
Pharmacotherapeutic group
Other medications for use in coughs and colds.
ATX code R05X.
Pharmacological properties
Pharmacodynamics
When applied externally, the drug has an irritating, distracting, anti-inflammatory, and antiseptic effect.
Pharmacological properties are due to the components that make up its composition:
menthol – dilates blood vessels, causes a feeling of cold, which is accompanied by an analgesic effect;
camphor – causes an irritating and analgesic effect;
thymol – an antiseptic with antibacterial and antifungal effects;
turpentine and eucalyptus oils – have an irritating effect when applied topically;
Nutmeg oil – inhibits the synthesis of prostaglandins.
Pharmacokinetics
The drug does not have a systemic effect.
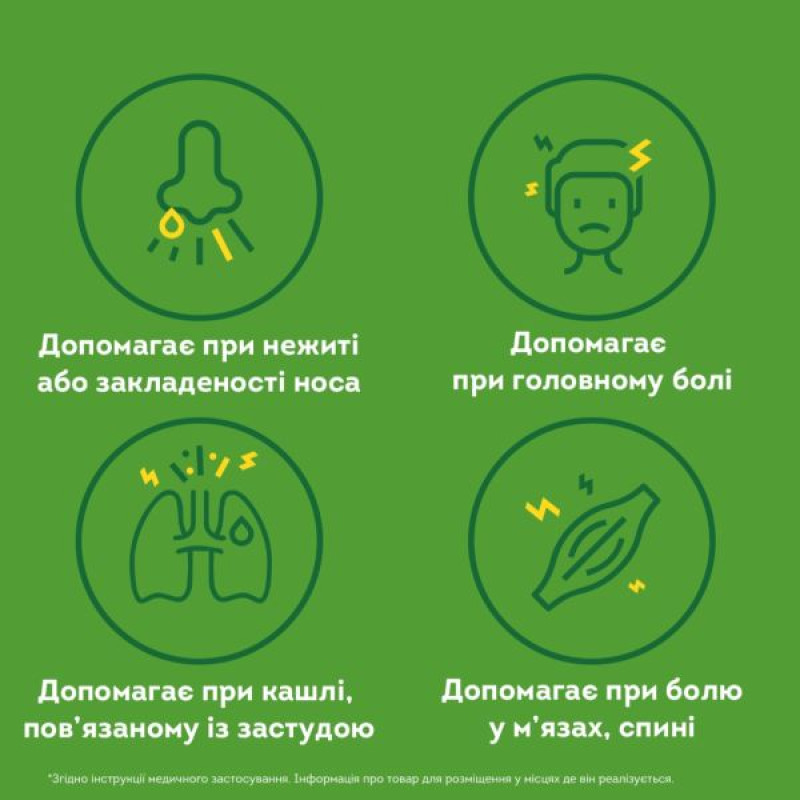
Indication
As part of the complex therapy of acute respiratory diseases accompanied by a runny nose, a feeling of nasal congestion, and cough.
Symptomatic therapy of muscle pain, back pain, headache.
Contraindication
Hypersensitivity/allergic reactions to the active substances or excipients of the drug.
Hypersensitivity to the components of the drug; bronchial asthma, whooping cough, pseudocroup, tendency to seizures; children under 3 years of age; irritation or violation of the integrity of the skin, including burns, eczema, dermatitis, pustular skin diseases.
Interaction with other medicinal products and other types of interactions
There are no reports of possible interactions of the combination of camphor, eucalyptus oil, menthol, nutmeg oil, thymol, and turpentine oil with other drugs.
Application features
For external use only!
Do not rub into the nostrils, avoid contact with eyes, nasal mucosa and oral cavity (if contact occurs, rinse with plenty of water). Do not apply to damaged skin!
If symptoms persist, worsen, or new symptoms appear, discontinue use of the drug and consult a doctor.
Use during pregnancy or breastfeeding
Due to the lack of experience with the use of DOCTOR MOM® ointment during pregnancy or breastfeeding, it is not recommended to prescribe the drug to this category of patients.
Ability to influence reaction speed when driving vehicles or other mechanisms
In case of adverse reactions from the nervous system (dizziness, agitation, convulsions), you should refrain from driving or working with other mechanisms.
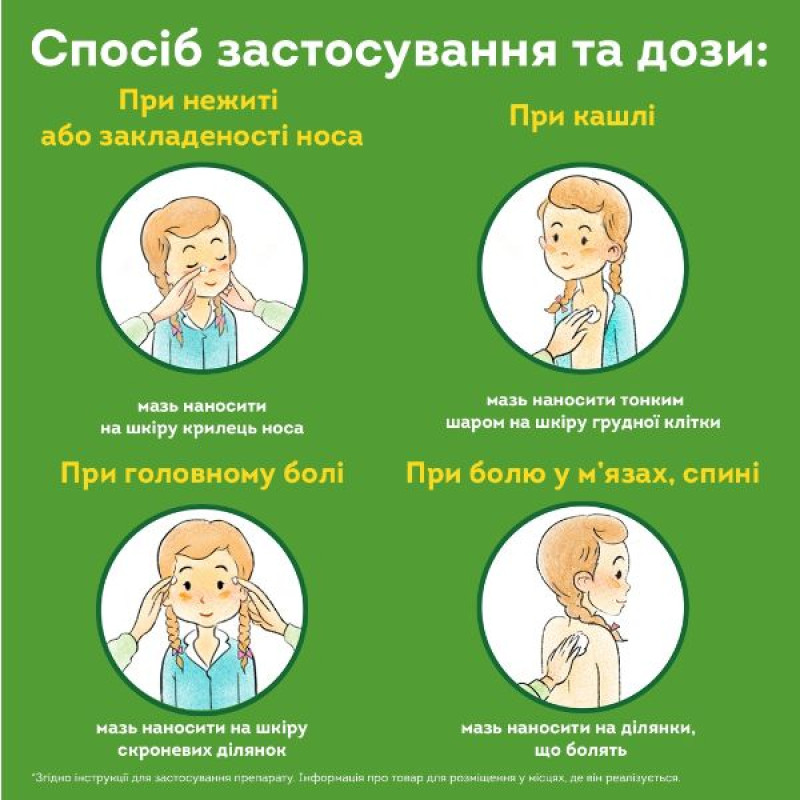
Method of administration and doses
Adults and children over 3 years of age should be prescribed DOCTOR MOM ointment up to 3 times a day.
| Fig. 1 | Fig. 2 |
| Fig. 3 | Fig. 4 |
For a runny nose or nasal congestion, apply a small amount of ointment carefully, without rubbing, to the skin of the alar area of the nose (see Fig. 1);
for coughs associated with a cold, apply a thin layer of ointment to the skin of the chest (in the sternum area) and rub in lightly (see Fig. 2);
for headaches, apply the ointment to the skin of the temporal areas (see Fig. 3);
For muscle and back pain, apply the ointment to the painful areas, rub in lightly, and cover these areas with a warm bandage (see Fig. 4).
Children
The efficacy and safety of the drug in children under 3 years of age have not been sufficiently studied. Children over 3 years of age should use with caution, on the recommendation and under the supervision of a physician.
Overdose
Possible increase in adverse reactions, feeling of intense heat and burning. In case of accidental ingestion, abdominal pain, nausea, vomiting, diarrhea, symptoms of central nervous system depression, dizziness, ataxia, convulsions, hot flashes, drowsiness, difficulty breathing are possible.
Treatment: wash off the applied drug with soap and water, gastric lavage, symptomatic therapy.
Adverse reactions
Allergic reactions are possible, including skin rashes, urticaria, itching, irritation of the skin and mucous membranes, redness, dermatitis, including contact dermatitis, especially in children.
On the part of the immune system: hypersensitivity (allergic reactions), anaphylactic shock.
From the nervous system: headache, dizziness, agitation, possible occurrence of convulsions caused by camphor.
On the part of the respiratory system: when using the drug, the likelihood and frequency of bronchospasm may increase, especially in children.
Skin and subcutaneous tissue disorders: allergic dermatitis.
If such reactions occur, treatment should be discontinued immediately.
Expiration date
3 years.
Storage conditions
Store at a temperature not exceeding 25 ° C in a place inaccessible to children. After use, the jar should be tightly closed with a lid.
Packaging
20 g in a plastic jar. 1 jar in a cardboard box.
Vacation category
Without a prescription.
Producer
Unique Pharmaceutical Laboratories (a division of J.B. Chemicals and Pharmaceuticals Ltd.).
Unique Pharmaceutical Laboratories (a division of JB Chemicals & Pharmaceuticals Ltd.).
Location of the manufacturer and its business address
Plot No. 304-308, G. I. D. C. Industrial Area, Panoli Town - 394 116, Bharuch District, India.
Plot no. 304-308, GIDC Industrial Area, City: Panoli - 394 116, Dist: Bharuch, India.
Applicant
Johnson & Johnson Ukraine LLC.
Applicant's location
01010, Kyiv, 32/2 Ostrozkyh Knyaziv St., Ukraine.
+38 (044) 498 0888
+38 (044) 498 7392
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.