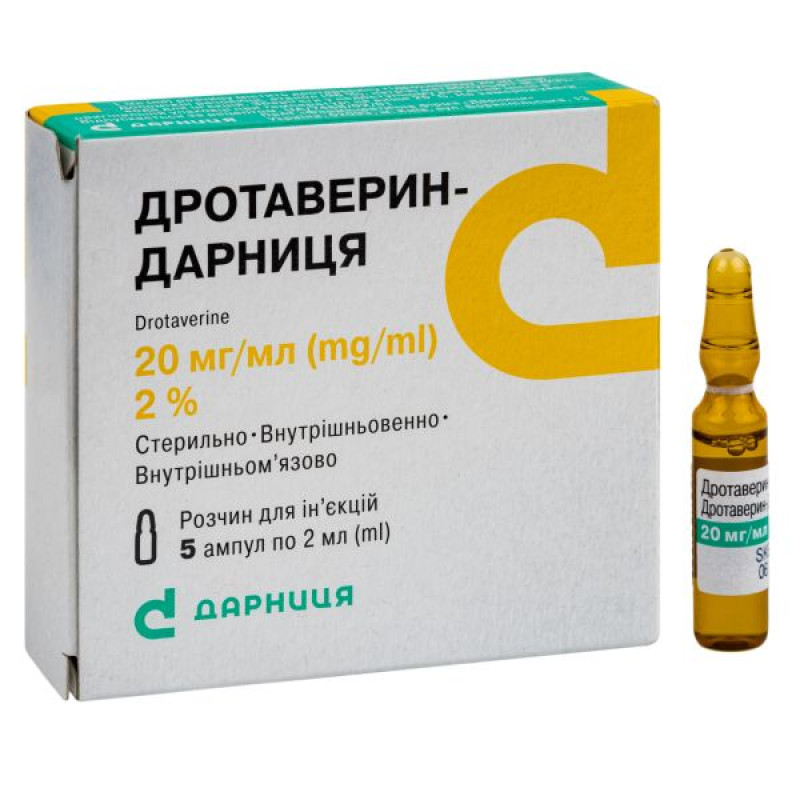
Drotaverin-Darnitsa solution for injection 2% ampoule 2 ml No. 5

Instructions Drotaverin-Darnitsa solution for injection 2% ampoule 2 ml No. 5
Composition
active ingredient: drotaverine;
1 ml of solution contains drotaverine hydrochloride 20 mg;
Excipients: ethanol (96%), sodium metabisulfite (E 223), water for injections.
Dosage form
Solution for injection.
Main physicochemical properties: transparent greenish-yellow or yellow liquid.
Pharmacotherapeutic group
Drugs used for functional gastrointestinal disorders. ATX code A03A D02.
Pharmacological properties
Pharmacodynamics
Drotaverine is an isoquinoline derivative that has an antispasmodic effect on smooth muscles by inhibiting the action of the enzyme phosphodiesterase IV (PDE IV), which causes an increase in the concentration of cAMP and, due to the inactivation of myosin light chain kinase (MLCK), leads to relaxation of smooth muscles.
In vitro, drotaverine inhibits the action of the enzyme PDE IV and inhibits the isoenzymes phosphodiesterase III (PDE III) and phosphodiesterase V (PDE V). PDE IV is of great functional importance for reducing the contractile activity of smooth muscles, therefore, selective inhibitors of this enzyme may be useful for the treatment of diseases accompanied by hypermotility, as well as various diseases in which gastrointestinal spasms occur.
In myocardial and vascular smooth muscle cells, cAMP is hydrolyzed mainly by the PDE III isoenzyme, therefore drotaverine is an effective antispasmodic that does not have significant side effects on the cardiovascular system and has a strong therapeutic effect on this system.
Drotaverine is effective in spasms of smooth muscles of both nervous and muscular origin. Drotaverine acts on the smooth muscles of the gastrointestinal, biliary, genitourinary and vascular systems, regardless of the type of their autonomic innervation. The drug enhances blood circulation in the tissues due to its ability to dilate blood vessels.
The effect of drotaverine is stronger than that of papaverine, absorption is faster and more complete, it binds less to blood plasma proteins. The advantage of drotaverine is also that, unlike papaverine, after its parenteral administration there is no such side effect as respiratory stimulation.
Pharmacokinetics
Drotaverine is rapidly and completely absorbed, to a greater extent (95-98%) binds to blood plasma proteins, especially albumin, gamma- and beta-globulins. After primary metabolism, 65% of the dose taken enters the bloodstream unchanged. It is metabolized in the liver. The half-life is 8-10 hours. Within 72 hours, drotaverine is almost completely excreted from the body, more than 50% is excreted in the urine and approximately 30% in the feces. Drotaverine is mainly excreted in the form of metabolites, it is not detected in the urine in unchanged form.
Indication
Smooth muscle spasms associated with biliary tract diseases such as cholecystolithiasis, cholangiolithiasis, cholecystitis, pericholecystitis, cholangitis, papillitis;
Smooth muscle spasms in urinary tract diseases: nephrolithiasis, urethrolithiasis, pyelitis, cystitis, bladder tenesmus.
As an adjunctive treatment (when the use of the drug in tablet form is not possible) for:
for spasms of smooth muscles of the gastrointestinal tract: gastric and duodenal ulcers, gastritis, cardio- and/or pylorospasm, enteritis, colitis; for gynecological diseases (dysmenorrhea).
Contraindication
Hypersensitivity to drotaverine or to other components of the drug (especially to sodium metabisulfite). Hypersensitivity to sodium disulfite. Severe hepatic, renal or heart failure (small cardiac output syndrome).
Interaction with other medicinal products and other types of interactions
When used simultaneously with levodopa, the antiparkinsonian effect of the latter may be reduced. This combination should be used with caution, as the antiparkinsonian effect of levodopa is reduced and rigidity and tremor are increased.
Application features
When administering the drug intravenously, the patient should be in a horizontal position due to the risk of collapse.
DROTAVERIN-DARNYTSYA should be used with caution in cases of arterial hypotension.
Caution should be exercised when parenterally administering the drug to pregnant women (see section "Use during pregnancy or lactation").
DROTAVERINE-DARNYTSYA contains metabisulfite, which may cause allergic-type reactions, including symptoms of anaphylactic shock and bronchospasm in sensitive patients, especially those with a history of asthma or allergies.
In case of hypersensitivity to sodium metabisulfite, parenteral administration of the drug should be avoided.
Ability to influence reaction speed when driving vehicles or other mechanisms
You should refrain from driving or operating other mechanisms that require increased attention while using the drug (especially intravenously).
Use during pregnancy or breastfeeding
As shown by the results of animal studies, oral administration of the drug did not cause any cases of teratogenicity and embryotoxicity. The drug should be used with caution during pregnancy. Do not use drotaverine during labor.
Due to the lack of relevant research data, the use of the drug during breastfeeding is not recommended.
Method of administration and doses
DROTAVERINE-DARNYTSYA should be administered intramuscularly to adults in a daily dose of 40-240 mg in 1-3 injections.
For acute colic, the drug should be administered intravenously slowly to adults at a dose of 40-80 mg.
Children
DROTAVERIN-DARNYTSYA should not be used in children.
Overdose
Symptoms: Overdose with drotaverine has been associated with cardiac rhythm and conduction disturbances, including complete bundle branch block and cardiac arrest, which can be fatal.
Treatment: symptomatic and supportive therapy. In case of overdose, the patient should be under close medical supervision.
Adverse reactions
From the cardiovascular system: arterial hypotension, tachycardia, arrhythmia, atrioventricular block.
From the nervous system: headache, dizziness, insomnia.
From the digestive tract: nausea, vomiting, constipation.
Immune system: hypersensitivity reactions, including urticaria, skin rashes, dermatosis, itching, flushing, fever, chills, increased body temperature, weakness, angioedema, bronchospasm, especially in patients with hypersensitivity to metabisulfite. There have been reports of cases of anaphylactic shock with fatal and non-fatal outcomes when using the injectable form.
DROTAVERINE-DARNYTSYA contains metabisulfite, which may cause allergic-type reactions, including symptoms of anaphylactic shock and bronchospasm in sensitive patients, especially those with a history of asthma or allergies.
General disorders: hypersensitivity reactions at the injection site.
Expiration date
3 years.
Storage conditions
Store out of the reach of children in the original packaging at a temperature not exceeding 25 ° C. Do not freeze.
Packaging
2 ml in an ampoule; 5 ampoules in a contour blister pack; 1 contour blister pack in a pack.
Vacation category
According to the recipe.
Producer
PrJSC "Pharmaceutical Company "Darnitsa".
Location of the manufacturer and its business address
Ukraine, 02093, Kyiv, Boryspilska St., 13.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.