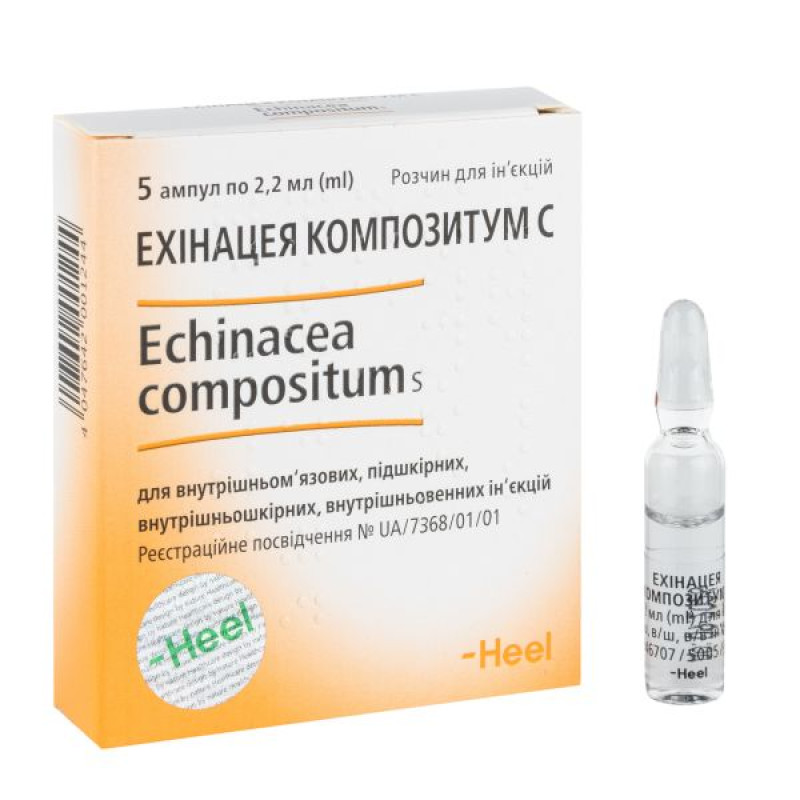
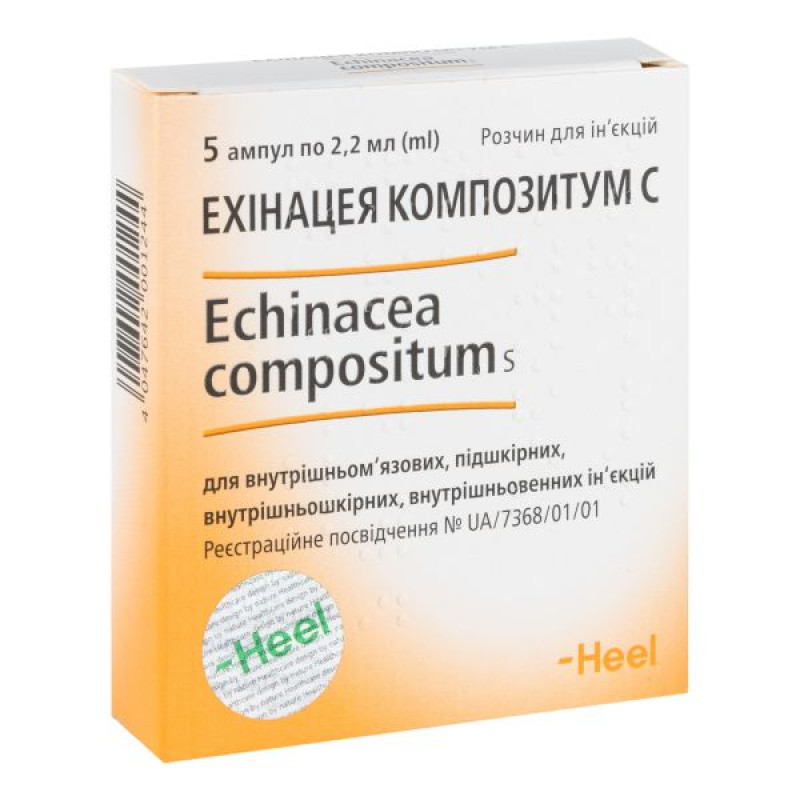
Echinacea compositum C solution for injection ampoule 2.2 ml No. 5
Instructions Echinacea compositum C solution for injection ampoule 2.2 ml No. 5
Composition
active substances: 2.2 ml of solution contain: Acidum arsenicosum D8 - 22 mg, Aconitum napellus D3 - 22 mg, Argentum nitricum D8 - 22 mg, Arnica montana D4 - 22 mg, Baptisia tinctoria D4 - 22 mg, Bryonia D6 - 22 mg, Cortisonum aceticum D13 - 22 mg, Echinacea D3 - 22 mg, Eupatorium perfoliatum D6 − 22 mg, Euphorbium D6 − 22 mg, Gelsemium sempervirens D6 − 22 mg, Grippeimpfstoff Nosode D13 − 22 mg, Hepar sulfuris D10 − 22 mg, Hydrargyrum bichloratum D8 − 22 mg, Lachesis D10 − 22 mg, Phosphorus D8 − 22 mg, Phytolacca americana D6 − 22 mg, Pulsatilla pratensis D8 − 22 mg, Pyrogenium Nosode D198 - 22 mg, Rhus toxicodendron D4 - 22 mg, Sanguinaria canadensis D4 - 22 mg, Staphylococcus Nosode D18 - 22 mg, Streptococcus haemolyticus Nosode D18 - 22 mg, Sulfur D8 - 22 mg, Thuja occidentalis D8 - 22 mg, Zincum metallicum D10 - 22 mg;
Excipients: sodium chloride, water for injections.
Dosage form
Solution for injection.
Main physicochemical properties: clear, colorless, odorless solution, practically free from particles.
Pharmacotherapeutic group
A complex homeopathic preparation.
Pharmacological properties
A drug with immunomodulatory, anti-inflammatory and detoxifying effects, which is based on the activation of the body's defenses and the normalization of impaired functions due to substances of plant, mineral and animal origin that are part of the drug.
Indication
Comprehensive treatment of acute and chronic inflammatory and purulent-infectious diseases of the mucous membranes, internal organs and skin, which occur with severe intoxication and frequent relapses.
Contraindication
Hypersensitivity to plants of the Asteraceae family (including Arnica, Echinacea), poison ivy or to other components of the drug.
Tuberculosis, sarcoidosis, inflammatory diseases of connective tissues (collagenoses), autoimmune diseases, multiple sclerosis, diseases of the blood system (leukemia), AIDS, HIV infections, immunosuppression or immunodeficiency of various etiologies, and other chronic viral diseases.
Interaction with other medicinal products and other types of interactions
Cases of exposure are unknown. The drug can be combined with other medications and treatment methods as prescribed by a doctor.
If medications with a pronounced hepatotoxic effect are used, the question of simultaneous administration with the drug “Echinacea compositum C” is decided by the doctor individually.
Application features
If symptoms persist or worsen, you should consult a doctor.
In case of liver disease or concomitant use of hepatotoxic drugs, the drug should be used only after consulting a doctor.
When using the drug for more than 4 weeks, it is necessary to monitor liver function indicators.
Use during pregnancy or breastfeeding
The drug is not used during pregnancy or breastfeeding due to the presence of Canadian bloodroot (Sanguinaria canadensis) in the composition.
Ability to influence reaction speed when driving vehicles or other mechanisms
Cases of exposure are unknown.
Method of administration and doses
A single dose, which is also a daily dose: adults and children over 12 years old - 2.2 ml; children from 2 to 3 years old - 0.6 ml, from 3 to 6 years old - 1.0 ml, from 6 to 12 years old - 1.5 ml. Apply 1-3 times a week in the form of intramuscular, subcutaneous, and if necessary - intravenous (jet) injections.
The course of treatment is 2 weeks.
If symptoms last more than 14 days, you should consult a doctor.
Instructions for opening the ampoule
Open carefully. Follow these instructions.
There is no need to cut the glass ampoule. Hold the ampoule with the top facing up at an angle; shake the liquid at the top of the ampoule downwards. Break off the top of the ampoule where the colored mark is.
Pour out the unused solution.
Children
The medicine is recommended for use in children aged 2 years and over.
Overdose
Not observed. Possible increase in adverse reactions.
Adverse reactions
In rare cases, gastrointestinal disorders or skin reactions may occur, including a few days after using the drug.
After using preparations containing echinacea extracts, reactions from the digestive tract (including nausea, abdominal pain); menstrual disorders; acne, skin rashes, itching, urticaria; agitation, sleep disturbances; in rare cases - facial swelling, difficulty breathing (dyspnea), dizziness and decreased blood pressure.
Increased salivation is possible - in this case, it is recommended to reduce the dose of the drug or discontinue its use.
During treatment with drugs containing Sanguinaria canadensis, isolated cases of increased serum transaminases and bilirubin levels, up to the appearance of drug-induced jaundice (drug-induced toxic hepatitis), have been observed, which decreased or normalized after discontinuation of the drug.
Reporting adverse reactions after registration of a medicinal product is of great importance. This allows monitoring of the benefit/risk ratio when using this medicinal product. Medical and pharmaceutical professionals, as well as patients or their legal representatives, should report all cases of suspected adverse reactions and lack of efficacy of a medicinal product via the Automated Information System for Pharmacovigilance at the link: https://aisf.dec.gov.ua.
Expiration date
5 years.
The expiration date determines the use of the drug until the last day of the month.
Storage conditions
This medicine does not require any special storage conditions. Keep out of the reach of children!
Incompatibility
Unknown.
Side effects
In rare cases, gastrointestinal disorders or skin reactions may occur, including a few days after using the drug.
During treatment with drugs containing Sanguinaria canadensis, isolated cases of increased serum transaminases and bilirubin levels, up to the appearance of drug-induced jaundice (drug-induced toxic hepatitis), have been observed, which decreased or normalized after discontinuation of the drug.
In rare cases, hypersensitivity reactions (up to anaphylactic reactions) may occur in individuals with hypersensitivity to plants of the Asteraceae family (including Arnica, Echinacea). Temporary reactions at the injection site may occur, including redness, swelling and pain.
After using preparations containing echinacea extracts, reactions from the digestive tract (including nausea, abdominal pain); menstrual disorders; acne, skin rashes, itching, urticaria; agitation, sleep disturbances; in rare cases - facial swelling, difficulty breathing (dyspnea), dizziness and decreased blood pressure.
Packaging
5 (5×1), 10 (5×2) or 100 (5×20) ampoules of 2.2 ml in a cardboard box.
Vacation category
According to the recipe.
Producer
Biologische Heilmittel Heel GmbH.
Location of the manufacturer and its business address
Dr. Reckeweg-Str. 2-4, 76532 Baden-Baden, Germany/Dr. Reckeweg-Str. 2-4, 76532 Baden-Baden, Germany.
Address
Dr. Reckeweg-Str. 2-4, 76532 Baden-Baden, Germany/Dr. Reckeweg-Str. 2-4, 76532 Baden-Baden, Germany.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.