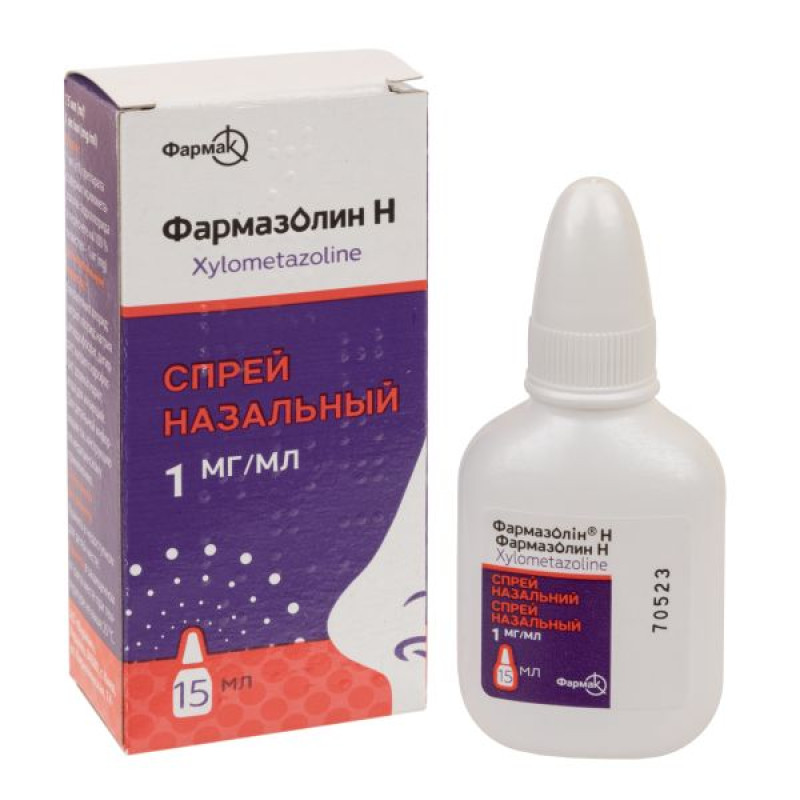
Farmazolin H nasal spray 1 mg/ml bottle 15 ml
Instructions Farmazolin H nasal spray 1 mg/ml bottle 15 ml
Composition
active ingredient: xylometazoline hydrochloride;
1 ml of the drug contains xylometazoline hydrochloride in terms of 100% substance 1 mg;
Excipients: benzalkonium chloride; sodium chloride; sodium dihydrogen phosphate, dihydrate; sodium hydrogen phosphate, dodecahydrate; water for injections.
Dosage form
Nasal spray.
Main physicochemical properties: clear colorless liquid.
Pharmacotherapeutic group
Anti-edematous and other drugs for local use in diseases of the nasal cavity. Sympathomimetics, simple drugs. ATX code R01A A07.
Pharmacological properties
Pharmacodynamics
Xylometazoline is a sympathomimetic agent that acts on α-adrenergic receptors.
Farmazolin® H causes narrowing of nasal blood vessels, reducing swelling and hyperemia of the nasal mucosa and its paranasal sinuses and, thus, improving nasal breathing in case of nasal and paranasal sinus congestion.
The drug begins to work a few minutes after application and lasts up to 12 hours (for example, throughout the night).
The drug is well tolerated, including by patients with sensitive mucous membranes. The drug does not reduce mucociliary function. Farmazolin® H has a balanced pH value within the limits characteristic of the nasal cavity.
In vitro studies have shown that xylometazoline reduces the infectious activity of human rhinovirus associated with the common cold.
Pharmacokinetics
With topical application, xylometazoline concentrations in blood plasma are so low that they cannot be determined by modern analytical methods.
Indication
Symptomatic treatment of nasal congestion in colds, hay fever, allergic rhinitis, sinusitis. To facilitate the outflow of secretions in diseases of the paranasal sinuses. Adjunctive therapy in cases of otitis media (to eliminate swelling of the mucous membrane). To facilitate rhinoscopy.
Contraindication
Hypersensitivity to the components of the drug; acute coronary disease, coronary asthma, hyperthyroidism, angle-closure glaucoma, dry rhinitis (rhinitis sicca) or atrophic rhinitis, surgical interventions with exposure of the dura mater or transsphenoidal hypophysectomy. Concomitant treatment with MAO inhibitors and within 2 weeks after their discontinuation.
Interaction with other medicinal products and other types of interactions
Monoamine oxidase inhibitors (MAOIs): Xylometazoline may potentiate the effects of monoamine oxidase inhibitors, which may cause hypertensive crisis. Xylometazoline is not recommended for patients who are taking or have taken MAOIs within the last two weeks.
Tri- and tetracyclic antidepressants: with simultaneous use of tri- or tetracyclic antidepressants and sympathomimetic drugs, the sympathomimetic effect of xylometazoline may be enhanced, therefore the simultaneous use of such drugs is not recommended.
When used together with β-blockers, it may cause bronchial spasm or a decrease in blood pressure.
Application features
The drug should not be used for more than 10 consecutive days. Prolonged or excessive use may lead to the recurrence of nasal congestion and/or atrophy of the nasal mucosa.
The recommended dose of the drug should not be exceeded, especially when treating children and the elderly.
The drug should be prescribed with caution to patients with cardiovascular diseases, high blood pressure, diabetes mellitus, prostatic hypertrophy, pheochromocytoma due to a possible systemic sympathomimetic effect. The drug, like other sympathomimetics, should be prescribed with caution to patients who have strong reactions to adrenergic agents, manifested in the form of sleep disorders, dizziness, tremor, cardiac arrhythmia, increased blood pressure. The drug contains benzalkonium chloride, which can cause irritation of the skin and mucous membranes.
Ability to influence reaction speed when driving vehicles or other mechanisms
The drug usually has no or negligible influence on the ability to drive or use machines.
Use during pregnancy or breastfeeding
The drug should not be used during pregnancy due to its potential vasoconstrictor effect.
There is no evidence of any adverse effects on the infant. It is not known whether xylometazoline is excreted in breast milk, so caution is required and the drug should be used during breastfeeding only as directed by a physician.
Fertility.
There are no adequate data on the effect of the drug on fertility. Since the systemic exposure to xylometazoline hydrochloride is very low, the likelihood of an effect on fertility is extremely low.
Method of administration and doses
Adults and children aged 12 and over
Administer 1 spray into each nostril 3 times a day.
The maximum daily dose is 3 injections.
The metered spray ensures accurate dosing and proper distribution of the solution over the surface of the nasal mucosa.
Before use, prime the dosing device by pressing it several times until the spray starts to come out into the air. The dosing device will be ready for immediate use for subsequent uses.
The spray should be applied as follows:
thoroughly clean the nose before using the drug; hold the bottle vertically, supporting the bottom with your thumb and placing the tip between two fingers; tilt the bottle slightly and insert the tip into the nostril; inject and simultaneously inhale lightly through the nose; after use, clean and dry the tip before closing the tip with the cap; to prevent infection, each bottle of the drug can be used by only one person.
The last application is recommended immediately before bedtime.
Children
The drug should be used in children over 12 years of age.
Overdose
Excessive topical application of xylometazoline hydrochloride or accidental ingestion may result in severe dizziness, excessive sweating, a significant decrease in body temperature, headache, bradycardia, arterial hypertension, respiratory depression, coma, and convulsions. Elevated blood pressure may change to low blood pressure. Young children are more sensitive to toxicity than adults.
All patients suspected of overdose should be given appropriate supportive measures and, if necessary, immediate symptomatic treatment under medical supervision. Medical care should include observation of the patient for several hours. In the case of severe overdose accompanied by cardiac arrest, resuscitation measures should last at least 1 hour.
Adverse reactions
Immune system disorders: hypersensitivity reactions, including angioedema, rash, itching.
From the nervous system: headache.
On the part of the organs of vision: temporary visual impairment.
Cardiovascular system: irregular or rapid heartbeat.
Respiratory, thoracic and mediastinal disorders: dryness or discomfort of the nasal mucosa.
Gastrointestinal: nausea.
General disorders and administration site conditions: burning sensation at the application site.
Expiration date
3 years.
Do not use the drug after the expiration date indicated on the package.
Storage conditions
Store in a place protected from light at a temperature not exceeding 25 ° C. Keep out of the reach of children.
Packaging
15 ml or 20 ml in a bottle. 1 bottle in a pack.
Vacation category
Without a prescription.
Producer
PJSC "Farmak".
Location of the manufacturer and its business address
Ukraine, 04080, Kyiv, Frunze St., 74.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.