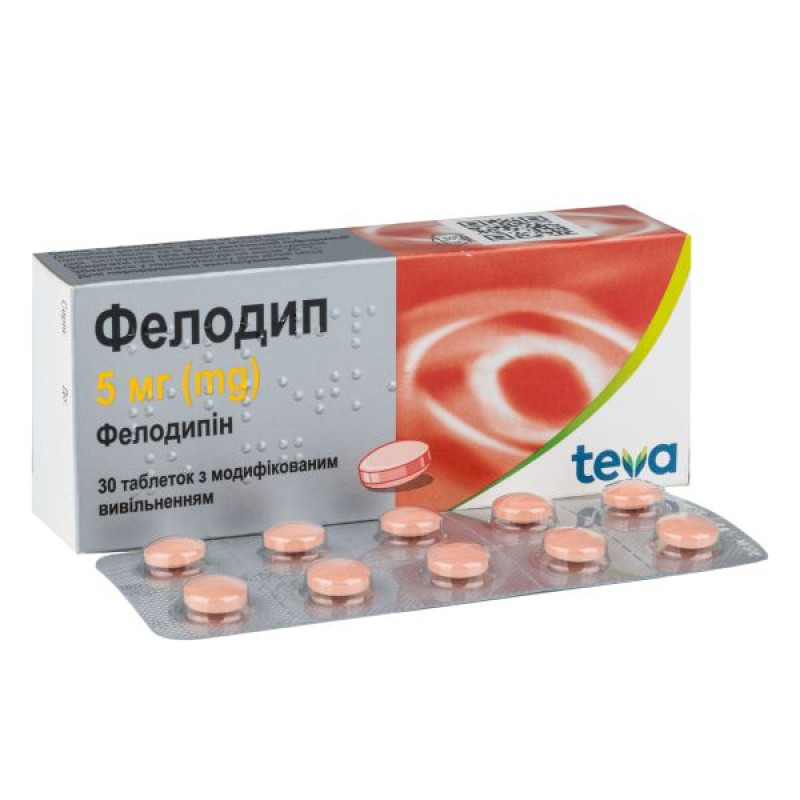
Felodip modified-release tablets 5 mg blister No. 30
Instructions for use Felodip modified-release tablets 5 mg blister No. 30
Composition
active ingredient: felodipine;
1 tablet contains 5 mg of felodipine;
excipients: lactose monohydrate, microcrystalline cellulose, hypromellose, povidone, propyl gallate, colloidal anhydrous silicon dioxide, magnesium stearate;
Tablet coating: hypromellose, red iron oxide (E 172), yellow iron oxide (E 172), titanium dioxide (E 171), talc, propylene glycol.
Dosage form
Modified-release tablets.
Main physicochemical properties: film-coated tablets, light pink in color, round, biconvex, 9 mm in diameter, with an imprint of "5" on one side.
Pharmacotherapeutic group
Selective calcium channel blockers with predominant vascular action. Dihydropyridine derivatives. ATX code C08C A02.
Pharmacological properties
Pharmacodynamics
Felodipine is a vasoselective calcium channel blocker that lowers blood pressure by reducing peripheral vascular resistance. In therapeutic doses, felodipine selectively acts on arteriolar smooth muscle and has no direct effect on cardiac contractility or conduction. The drug does not affect venous smooth muscle or adrenergic vasomotor mechanisms, and therefore felodipine is not associated with symptoms of orthostatic hypotension. Felodipine has its own moderate natriuretic and diuretic effects and, therefore, does not cause fluid retention.
Felodipine is effective in various degrees of arterial hypertension. It can be used as monotherapy or in combination with other antihypertensive drugs (e.g. β-blockers, diuretics or angiotensin-converting enzyme (ACE) inhibitors) to enhance the antihypertensive effect. Felodipine reduces systolic and diastolic blood pressure and can be prescribed in isolated systolic hypertension. Felodipine retains its antihypertensive efficacy also in combination with non-steroidal anti-inflammatory drugs.
Felodipine exhibits antianginal and antiischemic effects due to its effect on the balance between myocardial oxygen consumption and supply. Felodipine reduces coronary vascular resistance. Coronary blood flow and myocardial oxygen saturation also increase due to dilation of epicardial arteries and arterioles. Felodipine effectively prevents the formation and development of coronary vasospasm. The decrease in systemic blood pressure caused by felodipine reduces left ventricular afterload and reduces myocardial oxygen demand.
Felodipine improves exercise tolerance and reduces the frequency of attacks in patients with stable angina pectoris. In patients with stable angina pectoris, felodipine can be prescribed as monotherapy or in combination with ß-adrenoceptor blockers.
Felodipine is an effective agent that is well tolerated by adult patients, regardless of age, race, patients with comorbidities such as congestive heart failure, bronchial asthma and other obstructive pulmonary diseases, renal failure, diabetes mellitus, gout, hyperlipidemia, Raynaud's phenomenon, as well as patients after kidney transplantation. Felodipine does not affect glycemia and lipid profile.
Site and mechanism of action. The main pharmacodynamic feature of felodipine is its high degree of vascular selectivity. Myogenically active smooth muscle of resistance arterioles is particularly sensitive to the action of felodipine. Felodipine inhibits the electrical and contractile activity of vascular smooth muscle by acting on calcium channels in the cell membrane.
Hemodynamic effects. The primary hemodynamic effect of felodipine is a reduction in total peripheral vascular resistance, which leads to a decrease in blood pressure. This effect is dose-dependent. In general, the reduction in blood pressure is observed 2 hours after a single dose and lasts for at least 24 hours, and the T/P ratio (trough/peak) reaches values well above 50%. There is a positive relationship between the concentration of felodipine in the blood plasma, the reduction in peripheral vascular resistance and blood pressure.
Cardiac effects. At therapeutic doses, felodipine has no effect on cardiac contractility, atrioventricular conduction, or the refractory period of the atrioventricular node. Hypotensive therapy with felodipine is associated with significant regression of existing left ventricular hypertrophy.
Renal effects. Felodipine has a slight natriuretic and diuretic effect, as it reduces tubular sodium reabsorption. Felodipine does not affect the daily excretion of potassium or albumin. Renal vascular resistance is reduced after taking felodipine. Normal glomerular filtration rate (GFR) is not changed. In patients with impaired renal function, GFR may increase during treatment with felodipine.
In patients receiving cyclosporine after renal transplantation, felodipine reduces blood pressure, improves renal blood flow, and GFR. Felodipine may improve graft function in the early stages after transplantation.
Clinical efficacy: The Hypertension Optimal Treatment (HOT) trial examined the association of major cardiovascular events (such as acute myocardial infarction, stroke, and cardiovascular death) with diastolic blood pressure targets of ≤90 mmHg, ≤85 mmHg, and ≤80 mmHg and the achieved blood pressure, with felodipine as background therapy.
A total of 18,790 patients with arterial hypertension (diastolic blood pressure 100–115 mm Hg) aged 50–80 years were followed for a mean of 3.8 years (range 3.3–4.9). Felodipine was used as monotherapy or in combination with a β-blocker and/or an ACE inhibitor and/or a diuretic. Positive results of the study were a decrease in systolic and diastolic blood pressure to 139 and 83 mm Hg, respectively.
In the STOP-2 study (Swedish Trial in Old Patients with Hypertension-2), which included 6,614 patients aged 70 to 84 years, dihydropyridine calcium channel blockers (felodipine and isradipine) showed the same preventive effect on cardiovascular mortality and morbidity as other widely used classes of antihypertensive drugs – ACE inhibitors, β-blockers and diuretics.
Pharmacokinetics
Absorption. Felodipine is completely absorbed from the gastrointestinal tract after oral administration of modified-release tablets. Bioavailability in humans is approximately 15% and is independent of the dose taken within the therapeutic range. Due to the characteristics of the dosage form, the modified release of felodipine prolongs the absorption phase and ensures its uniform concentration in the blood plasma for 24 hours. When using the prolonged-release dosage form, the maximum level of felodipine in the blood plasma (tmax) is reached after 3-5 hours. In the case of simultaneous use of felodipine with fatty food, the rate of its absorption increases without changing the extent of absorption.
Distribution: Felodipine is 99% bound to plasma proteins, mainly albumin. The volume of distribution at steady state is 10 l/kg.
Metabolism. Felodipine is metabolized mainly in the liver by cytochrome P450 3A4 (CYP3A4), all its metabolites are inactive. Felodipine belongs to the drugs with high clearance, which averages 1200 ml/min. With prolonged use, no significant cumulation of the drug was observed.
In elderly patients and patients with impaired liver function, plasma concentrations of felodipine are higher than in younger patients. The pharmacokinetics of felodipine are not altered in patients with impaired renal function, including patients on hemodialysis.
Excretion. The half-life of felodipine is about 25 hours, and the equilibrium concentration is reached after 5 days. With prolonged use, cumulation of the active substance does not occur. Approximately 70% of the dose of the drug is excreted in the urine, and the rest in the feces in the form of metabolites. Less than 0.5% of the dose is excreted unchanged in the urine.
Linearity/non-linearity: Plasma concentrations are directly proportional to dose within the therapeutic range of 2.5–10 mg.
Indication
Arterial hypertension.
Preventive treatment of chronic stable angina.
Contraindication
Hypersensitivity to felodipine and other dihydropyridines (theoretical risk of cross-reactivity) or to other components of the medicinal product. Decompensated heart failure. Unstable angina. Acute myocardial infarction. Dynamic obstruction of the left ventricular outflow tract. Severe aortic/mitral stenosis.
Interaction with other medicinal products and other types of interactions
Felodipine is metabolized in the liver by cytochrome P450 3A4 (CYP3A4). Concomitant use of substances that interact with the cytochrome enzyme system
P450 3A4, may affect plasma levels of felodipine.
Enzyme interactions
Drugs that are inhibitors or inducers of the cytochrome P450 isoenzyme 3A4 may affect the plasma levels of felodipine.
Interactions leading to increased plasma concentrations of felodipine
Enzyme inhibitors such as cimetidine, ranitidine, erythromycin, itraconazole, ketoconazole, anti-HIV drugs/protease inhibitors (e.g. ritonavir), quinidine and some flavonoids found in grapefruit juice increase the plasma concentration of felodipine. When used together with the potent CYP3A4 inhibitor itraconazole, the Cmax and AUC of felodipine increased 8-fold and 6-fold, respectively. When used together with erythromycin, the Cmax and AUC of felodipine increased 2.5-fold, and cimetidine increased the Cmax and AUC of felodipine by approximately 55%. The combination of felodipine with potent CYP3A4 inhibitors should be avoided. In the event of clinically significant adverse events due to excessive exposure to felodipine resulting from the combination of the drug with potent CYP3A4 inhibitors, it is recommended to adjust the dose of felodipine and/or discontinue the use of the CYP3A4 inhibitor.
Enzyme inducers such as phenytoin, carbamazepine, rifampicin, barbiturates, efavirenz, nevirapine and St. John's wort (Hypericum perforatum) may reduce plasma concentrations of felodipine, and patients taking these drugs may require higher doses. When felodipine was administered with carbamazepine, phenytoin or phenobarbital, the Cmax of felodipine was reduced by 82% and the AUC by 96%. Combinations of felodipine with potent CYP3A4 inducers should be avoided.
In case of insufficient clinical efficacy due to decreased felodipine concentrations when used in combination with strong CYP3A4 inducers, it is recommended to adjust the felodipine dose and/or discontinue the use of the CYP3A4 inducer.
Additional interactions
Felodipine may increase tacrolimus concentrations. In the case of concomitant use of felodipine and tacrolimus, it is necessary to monitor the concentration of tacrolimus in the blood plasma and adjust its dose accordingly.
Felodipine does not affect the plasma concentration of cyclosporine.
Grapefruit juice increases the plasma levels and bioavailability of felodipine due to the presence of flavonoids in it, so it should not be used together with felodipine.
Antihypertensive drugs prolong the hypotensive effect of felodipine.
Sympathomimetics reduce the effect of felodipine.
No dose adjustment is required when felodipine is used concomitantly with digoxin.
Felodipine does not affect the free fraction of other drugs that are highly bound to plasma proteins, such as warfarin.
Application features
The efficacy and safety of felodipine in the treatment of hypertensive crisis have not been studied.
Like other vasodilators, Felodipin may rarely cause significant hypotension with tachycardia, which in susceptible patients may lead to myocardial ischemia.
Felodipine is metabolized in the liver. Therefore, higher therapeutic concentrations and response to treatment can be expected in patients with significantly reduced liver function (see section "Method of administration and dosage").
Concomitant use of potent inducers or inhibitors of CYP3A4 may result in significant reduction or increase in plasma levels of felodipine, respectively. Therefore, such combinations should be avoided (see section 4.5).
Mild gingival hyperplasia has been reported in patients with severe gingivitis/periodontitis. This can be prevented by maintaining good oral hygiene.
Felodip should be administered with caution to patients with severe left ventricular dysfunction.
The drug contains lactose, therefore patients with rare hereditary forms of galactose intolerance, lactase deficiency or glucose-galactose malabsorption should not use this drug.
Ability to influence reaction speed when driving vehicles or other mechanisms
Felodipine has minor or moderate influence on the ability to drive and use machines. If patients experience headache, nausea, dizziness or weakness while taking the drug, their reaction time may be impaired. Particular caution should be exercised at the beginning of therapy.
Use during pregnancy or breastfeeding
Pregnancy
Felodipine is contraindicated during pregnancy. Nonclinical reproductive toxicity studies have shown effects on fetal development related to the pharmacological action of felodipine.
Breast-feeding
Felodipine may pass into breast milk, but it is not known whether it has any adverse effects on the newborn. Due to limited data on the safety of the drug in breastfed infants, felodipine should not be used during breastfeeding.
Fertility
There are no data on the effects of felodipine on fertility. Studies in rats have shown some effects on fetal development and no effects on fertility at therapeutic doses.
Method of administration and doses
Adults:
Arterial hypertension.
The dosage regimen is always determined individually.
Therapy is initiated at a dose of 5 mg once daily. Depending on clinical response, the dose may be reduced to 2.5 mg or increased to 10 mg daily. If necessary, another antihypertensive agent may be added. The standard maintenance dose is 5 mg once daily.
Angina pectoris.
The dosage regimen is always determined individually.
Therapy begins with a dose of 5 mg once a day. If necessary, this dose can be increased to 10 mg once a day.
Felodipine can be used in combination with β-blockers, ACE inhibitors or diuretics. The hypotensive effects of these drugs may be additive, so combination therapy should be used with caution to avoid hypotension.
Elderly patients: Treatment should be started with the lowest possible dose of the drug.
Patients with renal impairment: Patients with renal impairment do not require dose adjustment.
Method of administration: the drug is best taken in the morning, before meals or after a light breakfast that does not contain a lot of fat and carbohydrates. The tablets should not be chewed, divided or crushed to preserve their modified-release properties. The tablets should be swallowed whole with water.
Children
Given the limited experience in pediatric practice, the drug should not be prescribed to children.
Overdose
Symptoms: Overdose may cause excessive peripheral vasodilation with marked hypotension, which may sometimes be accompanied by bradycardia.
Treatment. If possible, use activated charcoal, gastric lavage, if no more than 1 hour has passed after taking an excessive dose. In case of severe arterial hypotension, symptomatic treatment is indicated. The patient should be given a horizontal position with raised legs. In case of bradycardia, intravenously administer atropine 0.5–1 mg. If this is not enough, it is necessary to replenish the blood plasma volume by infusion, for example, glucose, 0.9% sodium chloride solution, dextran. If the measures taken have not led to the normalization of the clinical condition, sympathomimetics with a predominant effect on α1-adrenoreceptors can be used.
Adverse reactions
Like other calcium channel blockers, the drug may cause facial flushing, headache, palpitations, dizziness and fatigue. Most of these reactions are dose-dependent and most often occur at the beginning of treatment or when the dose is increased. They are usually transient. Depending on the dose, ankle swelling may also occur, which is a consequence of precapillary vasodilation and not a tendency to retain fluid in the body. Gingival hyperplasia may occur in patients with gingivitis or periodontitis, which can be prevented by maintaining careful oral hygiene.
Immune system disorders: Hypersensitivity reactions (e.g. urticaria and angioedema).
Nervous system: headache, sleep disturbances, drowsiness, dizziness, paresthesia, anxiety, irritability, confusion, depression.
From the respiratory system: shortness of breath, nosebleeds.
Cardiovascular system: worsening of angina pectoris (especially at the beginning of treatment). Predominantly in patients with symptomatic ischemic heart disease: syncope, palpitations, tachycardia, myocardial infarction, hot flashes, peripheral edema (the degree of edema in the ankles depends on the dose), arterial hypotension.
Reproductive system: impotence/sexual dysfunction.
From the urinary system: frequent urination.
Gastrointestinal: nausea, gingivitis, gingival hyperplasia, abdominal pain, vomiting, periodontitis, diarrhea, constipation, dry mouth.
Hepatobiliary system: increased liver enzyme levels, cholestatic hepatitis.
Skin: hyperemia, skin rash, itching, photosensitivity, angioedema, erythema multiforme, erythema nodosum, leukocytoclastic vasculitis.
Musculoskeletal and connective tissue disorders: arthralgia, myalgia, muscle tremor.
Systemic disorders and local reactions: increased fatigue, increased body temperature.
Expiration date
4 years.
Storage conditions
Store at a temperature not exceeding 25 ˚C. Keep out of the reach of children.
Packaging
10 tablets in a blister; 3 blisters in a box.
Vacation category
According to the recipe.
Producer
Teva Czech Industries s.r.o.
Merkle GmbH.
Location of the manufacturer and its business address
Ostravska Street 305/29, Komarov, 747 70 Opava, Czech Republic.
Ludwig-Merkle-Strasse 3, 89143 Blaubeuren, Germany.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.