Ftalazol tablets 500 mg blister No. 100
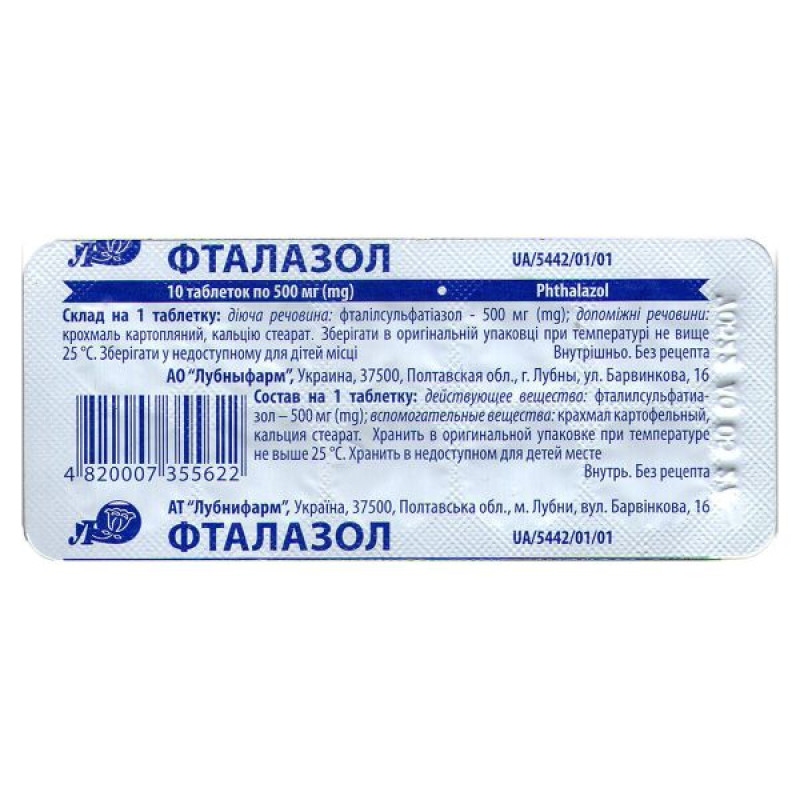
Instructions for use Ftalazol tablets 500 mg blister No. 100
Composition
active ingredient: phthalylsulfathiazole;
1 tablet contains phthalylsulfathiazole 500 mg;
excipients: potato starch, calcium stearate.
Dosage form
Pills.
Main physicochemical properties: solid, regular-shaped round cylinders, the upper and lower surfaces of which are flat, the edges of the surfaces are beveled with a score and a chamfer for division, white or white with a slightly yellowish tint.
Pharmacotherapeutic group
Antimicrobial agents used for intestinal infections. Sulfonamides.
ATX code A07A B02.
Pharmacological properties
Pharmacodynamics.
"Phtalazol" is a broad-spectrum antimicrobial sulfonamide drug containing the active ingredient phthalylsulfathiazole.
The drug is active against gram-positive and gram-negative microorganisms - pathogens of intestinal infections. It exhibits a bacteriostatic effect due to the disruption of the formation of growth factors of microorganisms - folic and dihydrofolic acids, necessary for the synthesis of purines and pyrimidines in microorganisms.
It also has an anti-inflammatory effect due to its ability to limit leukocyte migration, reduce the total number of migrating cellular elements, and partially stimulate the production of glucocorticoids.
Pharmacokinetics.
Phthalylsulfathiazole is practically not absorbed from the digestive tract, bioavailability is 5-10%. The main amount of it is retained in the intestine, where the active sulfonamide part of the molecule is cleaved - sulfathiazole. Part of the drug that was absorbed is acetylated in the liver and excreted in the urine. The high concentration of sulfathiazole in the intestine, taking into account the specific bacteriostatic activity of the drug against intestinal microflora, determines the high effectiveness of phthalylsulfathiazole in intestinal infections.
Indication
Acute dysentery (shigellosis), chronic dysentery in the acute phase, colitis, enterocolitis, gastroenteritis; prevention of infectious complications during intestinal surgeries.
Contraindication
Hypersensitivity to phthalylsulfathiazole, sulfonamide drugs and/or other components of the drug. Graves' disease, blood diseases, acute hepatitis.
Interaction with other medicinal products and other types of interactions
Depending on the nature of the disease, "Ftalazol" can be used in combination with antibiotics (enhanced antimicrobial action is observed). Simultaneously with the drug, it is advisable to prescribe well-absorbed sulfonamides (sulfadimezin, etazol, etazol-sodium).
Incompatible with para-aminosalicylic acid, salicylates, diphenyl (enhancement of the toxic effect of phthalylsulfathiazole), oxacillin (reduction of oxacillin activity), nitrofurans (increased risk of anemia and methemoglobinemia), male and female sex hormone preparations (suppression of gonadal function), calcium chloride and vitamin K (reduction of blood clotting).
"Ftalazol" cannot be used simultaneously with enterosorbents and laxatives.
Myelotoxic drugs enhance the manifestations of the drug's hematotoxicity.
Application features
The drug should be taken with caution in patients with nephrosis and nephritis.
It is advisable to use B vitamins simultaneously with the drug "Ftalazol", since due to the inhibition of the growth of E. coli, the synthesis of vitamins of this group decreases.
Use during pregnancy or breastfeeding
Do not use during pregnancy.
Phthalylsulfathiazole passes into breast milk and can cause kernicterus in children, as well as hemolytic anemia in children with glucose-6-phosphate dehydrogenase deficiency, therefore "Phthalazole" is not prescribed during breastfeeding.
Ability to influence reaction speed when driving vehicles or other mechanisms
Does not affect.
Method of administration and doses
Used internally by adults and children over 3 years of age.
Adults and children aged 12 and over.
In acute dysentery, it is used: in the first 2 days of treatment - 6 g per day (1 g every 4 hours), on the 3rd-4th day - 4 g per day (1 g every 6 hours), on the 5th-6th day - 3 g per day (1 g every 8 hours). The course dose is 25-30 g.
5-6 days after the first course of treatment, a second course is administered: the first 2 days - 1 g every 4 hours (at night - after 8 hours), a total of 5 g per day; 3-4th day - 1 g every 4 hours (not used at night), a total of 3 g per day. The course dose is 21 g (with a mild course of the disease, the course dose can be reduced to 18 g).
Maximum doses: single dose – 2 g, daily dose – 7 g.
When treating other diseases, the drug is used in the first 2-3 days at 1-2 g every 4-6 hours, in the next 2-3 days - 500 mg -1 g.
Children aged 3 to 12 years.
In the treatment of other diseases, the drug is used on the 1st day at the rate of 100 mg/kg of body weight per day. It is taken in equal doses every 4 hours with a break for the night. In the following days, 250-500 mg is used every 6-8 hours. The course of treatment is up to 7 days.
If the child cannot swallow the tablet, it should be crushed and dissolved in a small amount of boiled, cooled water.
Children.
The drug is not prescribed for children under 3 years of age.
Overdose
Symptoms: macrocytosis and pancytopenia may occur due to folic acid deficiency. This can be prevented by prescribing folic acid or calcium folinate. Typical adverse reactions may occur.
Treatment: drug withdrawal, symptomatic therapy.
Adverse reactions
The drug is well tolerated in most cases. Systemic adverse reactions typical of sulfonamides occur rarely due to the low absorption of the active substance. Allergic reactions are possible, including fever, rash, itching;
- from the hematopoietic system: agranulocytosis, aplastic anemia;
- others: hypovitaminosis of B vitamins (thiamine, riboflavin, nicotinic acid) due to suppression of intestinal microflora.
Expiration date
5 years.
Storage conditions
Store in the original packaging at a temperature not exceeding 25ºС.
Keep out of reach of children.
Packaging
10 tablets in blisters.
Vacation category
Without a prescription.
Producer
Lubnyfarm PJSC.
Location of the manufacturer and its business address.
Ukraine, 37500, Poltava region, Lubny, Barvinkova st., 16.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.