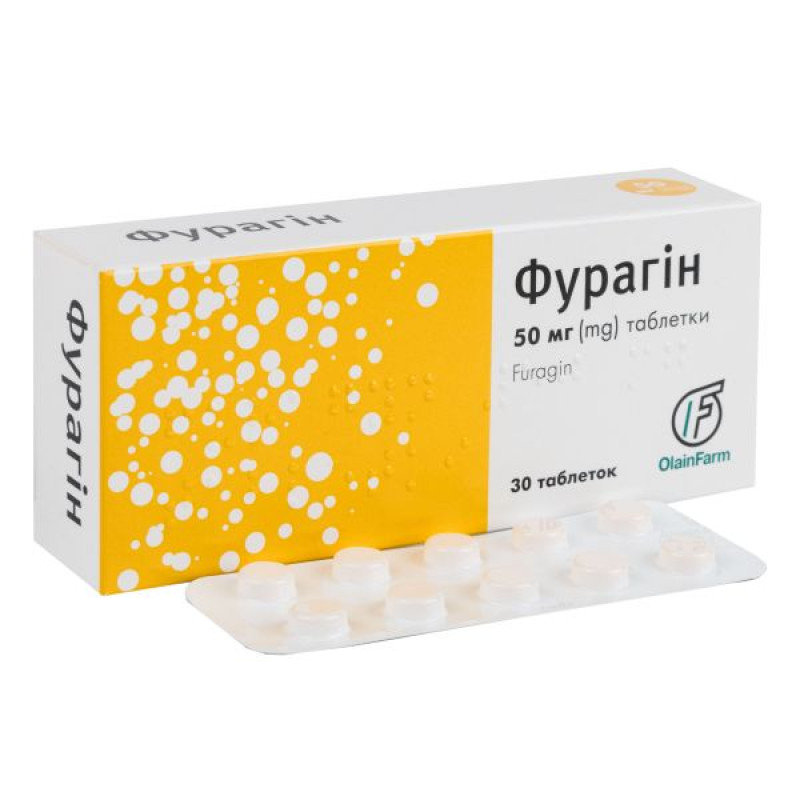
Furagin tablets 50 mg No. 30
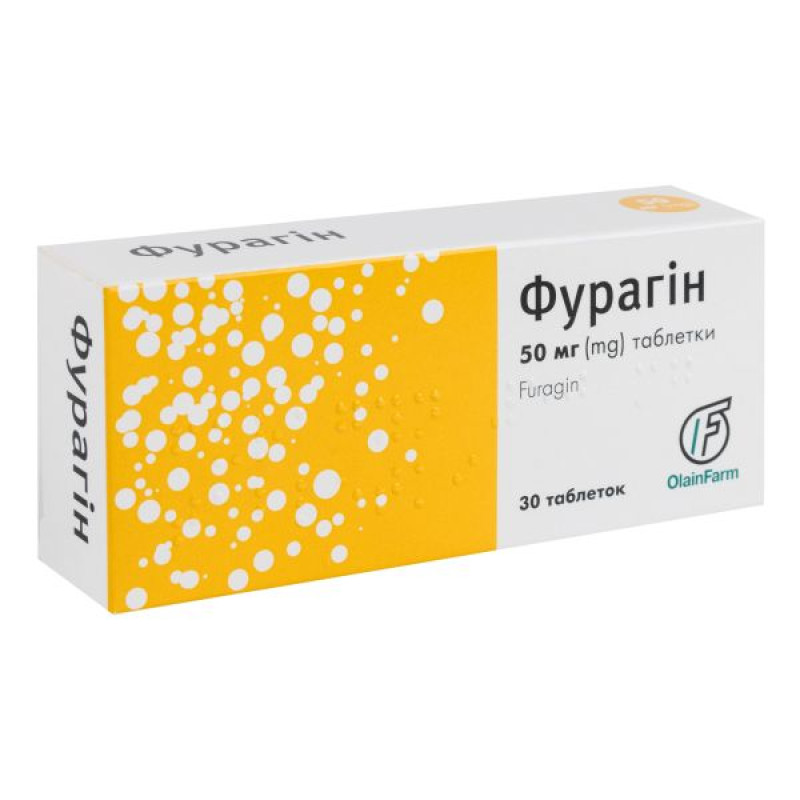
Instructions Furagin tablets 50 mg No. 30
Composition
active ingredient: furagin;
1 tablet contains furagin 50 mg;
excipients: lactose monohydrate; sugar; modified corn starch; stearic acid; polysorbate.
Dosage form
Pills.
Main physicochemical properties: flat-cylindrical tablets with a bevel from yellow to yellow with an orange tint.
Pharmacotherapeutic group
Antimicrobials for systemic use. Nitrofuran derivatives. Furazidin.
ATX code J01X E03.
Pharmacological properties
Pharmacodynamics
Furagin is a nitrofuran antibacterial agent with a bacteriostatic effect. It is effective against gram-positive (Staphyloccocus epidermidis, Staphyloccocus aureus, Staphyloccocus faecalis), gram-negative ((Enterobacteriaceae, Klebsiella spp, Eschrichia coli) bacteria. The high bacteriostatic activity of furagin is associated with the presence of an aromatic nitro group. Resistance to furagin develops slowly. Furagin inhibits the enzyme systems of microorganisms, as well as other biochemical processes in the bacterial cell, which in turn causes disruption of the cytoplasmic membrane and cell wall of the bacterium.
Pharmacokinetics.
Absorption. Furagin is well absorbed from the digestive tract. Absorption of the drug occurs mainly from the distal part of the small intestine by passive diffusion (exceeds absorption from the proximal part several times). After a single dose of 200 mg, the maximum concentration of furagin is reached in the blood plasma after 30 minutes, remains at this level for 1 hour, then slowly decreases. The bacteriostatic concentration of furagin in the blood plasma is maintained for 8−12 hours. Furagin binds to blood plasma proteins.
Metabolism/Elimination: 10% of the administered dose is biotransformed in the liver and kidneys. In cases of impaired renal function, biotransformation of a greater part of the administered dose occurs.
The half-life of furagin is short (about 1 hour). Furagin is excreted by the kidneys, mainly by tubular secretion (85%). 8-13% of furagin in unchanged form enters the urine, where its concentration is many times higher than the minimum inhibitory concentration for most sensitive bacteria. The maximum concentration of furagin in urine is 5.7 μg/ml.
Furagin penetrates well through the placental barrier.
Indication
Acute and chronic urinary tract infections: pyelonephritis, cystitis, urethritis; prostatitis; postoperative infections of the genitourinary system.
Contraindication
Hypersensitivity to furagin, to nitrofuran derivatives or to excipients of the drug;
– severe renal failure (creatinine clearance less than 30 ml/min);
− severe liver failure;
– polyneuropathy (including diabetic);
– glucose-6-phosphate dehydrogenase deficiency (risk of hemolysis);
– porphyria (diseases caused by impaired metabolism of hemoglobin breakdown products);
– rare congenital galactose intolerance, lactase deficiency or glucose-galactose malabsorption;
– sucrase/isomaltase/lactase deficiency, fructose/lactose intolerance.
Contraindicated in patients undergoing hemodialysis or peritoneal hemodialysis.
Contraindicated in children (under 18 years of age).
Do not use during pregnancy and breastfeeding.
Furagin is not recommended for urosepsis and kidney parenchymal infections.
Interaction with other medicinal products and other types of interactions
Agents that alkalize urine reduce the therapeutic effect of Furagin (accelerate the excretion of Furagin in the urine).
Agents that acidify urine (acids, including ascorbic acid, as well as calcium chloride) increase the concentration of Furagin in the urine (slowing down its excretion in the urine) and thus enhance the therapeutic effect of the drug, but at the same time increase the risk of increased toxicity.
Concomitant use with chloramphenicol, ristomycin and sulfonamides increases the inhibition of hematopoiesis.
Due to the antagonism of the action of Furagin with quinolones (nalidixic acid, oxolinic acid, norfloxacin), the simultaneous use of these drugs should be avoided.
The use of probenecid and sulfinpyrazone reduces the excretion of furagin, which increases the risk of developing undesirable side effects and toxicity.
Simultaneous use of Furagin and antacids (containing magnesium trisilicate) reduces the absorption of Furagin.
In case of renal failure, it is not recommended to use Furagin simultaneously with aminoglycosides.
The antibacterial effect of Furagin is significantly enhanced when used simultaneously with antibiotics (penicillins and cephalosporins), and it combines well with tetracycline and erythromycin.
You should not drink alcohol during treatment, as alcohol may increase the severity of side effects (increased heartbeat, pain in the heart area, headache, nausea, vomiting, seizures, low blood pressure, fever, anxiety).
Application features
The drug should be used with caution in the following cases:
– anemia;
– deficiency of B vitamins and folic acid;
With prolonged use of Furagin, peripheral neuropathy (pain, impaired sensitivity in the area of the corresponding nerve) may develop. If symptoms of neuropathy develop, the drug should be discontinued.
In diabetes, the drug may cause polyneuropathy.
The use of Furagin is not recommended for urosepsis and kidney parenchymal infections.
Experimental studies and clinical observations of patients have shown that nitrofurans adversely affect testicular function, which is manifested by a decrease in the number of sperm and ejaculate, a decrease in sperm motility, and pathological changes in their morphology.
When using Furagin, diarrhea may occur, caused by the drug's suppression of the normal microflora of the large intestine.
In rare cases, pseudomembranous colitis may develop, which is caused by the suppression of the natural microflora of the rectum and the reproduction of Clostridium difficile. In mild cases of pseudomembranous colitis, it is enough to stop taking the drug. During appropriate therapy, do not take drugs that slow intestinal peristalsis. Taking Furagin with food reduces the risk of colitis, without significantly affecting the absorption of the drug.
Laboratory tests of patients who have used Furagin may give a false-positive reaction for the presence of glucose in the urine if the copper reduction method is used for determination. Furagin does not affect the results of the determination of glucose in the urine by the enzymatic method.
In the case of long-term therapy, regular blood tests (leukocyte count) should be performed, liver and kidney function should be monitored, and lung function should be checked, especially in patients over 65 years of age.
The medicinal product contains sugar, which should be taken into account when treating patients with diabetes.
If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine.
To prevent neuritis, it is advisable to simultaneously take antihistamines and B vitamins (nicotinamide, thiamine).
Use during pregnancy or breastfeeding
Furagin is contraindicated during pregnancy or breastfeeding.
Ability to influence reaction speed when driving vehicles or other mechanisms
Usually, the drug does not affect the reaction speed when driving or working with other mechanisms, but caution should be exercised in individuals who experience dizziness, drowsiness, or other side effects from the central nervous system during treatment.
Method of administration and doses
Furagin should be taken orally, immediately after meals, with plenty of water.
Adults: 100-200 mg (2-4 tablets) 2-3 times a day. The course of treatment is 7-10 days, depending on the severity of the disease, the effectiveness of treatment, as well as the functional state of the kidneys. If necessary, the course of treatment is repeated after 10-15 days.
The maximum daily dose is 600 mg.
If a dose is missed, the next dose should be taken as soon as the patient remembers.
You should not take a double dose of the drug to make up for a missed dose.
Children
The medicine should not be used in children.
Overdose
Typically, toxic manifestations are possible in patients with impaired renal function.
Symptoms: headache, dizziness, depression, peripheral polyneuritis, allergic reactions (urticaria, angioedema, bronchospasm), nausea, vomiting, hemolytic anemia (in patients with glucose-6-phosphate dehydrogenase deficiency), megaloblastic anemia, rarely - liver dysfunction.
Treatment: discontinuation of the drug, drinking plenty of fluids, use of enterosorbents, antihistamines, B vitamins (thiamine bromide). Symptomatic therapy. In severe cases, hemodialysis.
There is no specific antidote.
Adverse reactions
Furagin, like other medicines, can cause side effects that do not occur in all patients.
Frequency of adverse reactions according to MedDRA classification: very common (≥ 1/10); common (≥ 1/100 to <1/10); uncommon (≥ 1/1000 to <1/100); rare (≥ 1/10000 to <1/1000); very rare (<1/10000), including isolated cases; unknown (cannot be estimated from the available data).
From the blood and lymphatic system: very rarely - hematopoietic disorders (agranulocytosis, thrombocytopenia, aplastic anemia).
Immune system disorders: hypersensitivity reactions including pruritus, rash, urticaria, angioedema.
From the nervous system: infrequently - dizziness, drowsiness; rarely - peripheral neuropathy.
On the part of the organs of vision: rarely - visual impairment.
Acute pulmonary reaction develops uncontrollably. It is manifested by severe shortness of breath, fever, chest pain, cough. with or without sputum, eosinophilia (increased number of eosinophil granulocytes in the blood). It has been reported that skin rashes, itching, urticaria, myalgia (muscle pain), angioedema (swelling of the face, neck, tissues of the oral cavity and larynx) occur simultaneously with acute pulmonary reaction. The basis of acute pulmonary reaction is a hypersensitivity reaction, which can develop within several hours, less often within minutes. Acute pulmonary reaction disappears when the drug is discontinued. Chronic pulmonary reaction may develop after a long period of time after discontinuation of treatment with nitrofurans (including Furagin). Characterized by gradual onset of dyspnea, tachypnea, unstable fever, eosinophilia, progressive cough, and interstitial pneumonitis and/or pulmonary fibrosis.
From the digestive tract: infrequently - decreased appetite, nausea; rarely - vomiting, diarrhea; very rarely - pancreatitis.
Skin and subcutaneous tissue disorders: rarely - papular rashes, itching; very rarely - angioedema, urticaria, exfoliative dermatitis, erythema multiforme.
Musculoskeletal and connective tissue disorders: very rarely - arthralgia (joint pain).
From the hepatobiliary system: very rarely - cholestatic jaundice, hepatitis.
General disorders: rarely - weakness, increased body temperature, temporary hair loss.
Furagin stains urine dark yellow or brown.
To reduce side effects, it is recommended to take B vitamins, antihistamines, and drink plenty of fluids. In case of severe side effects, the dose should be reduced or the drug should be discontinued.
If you experience side effects while taking the medicine that are not listed in the instructions for medical use, you should inform your doctor.
Expiration date
5 years.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 °C.
Keep out of reach of children.
Packaging
10 tablets in a blister. 3 blisters in a cardboard pack.
Vacation category
According to the recipe.
Producer
JSC "Olainfarm" / JSC "Olainfarm".
Location of the manufacturer and its business address
5 Rupnicu street, Olaine, LV-2114, Latvia.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.