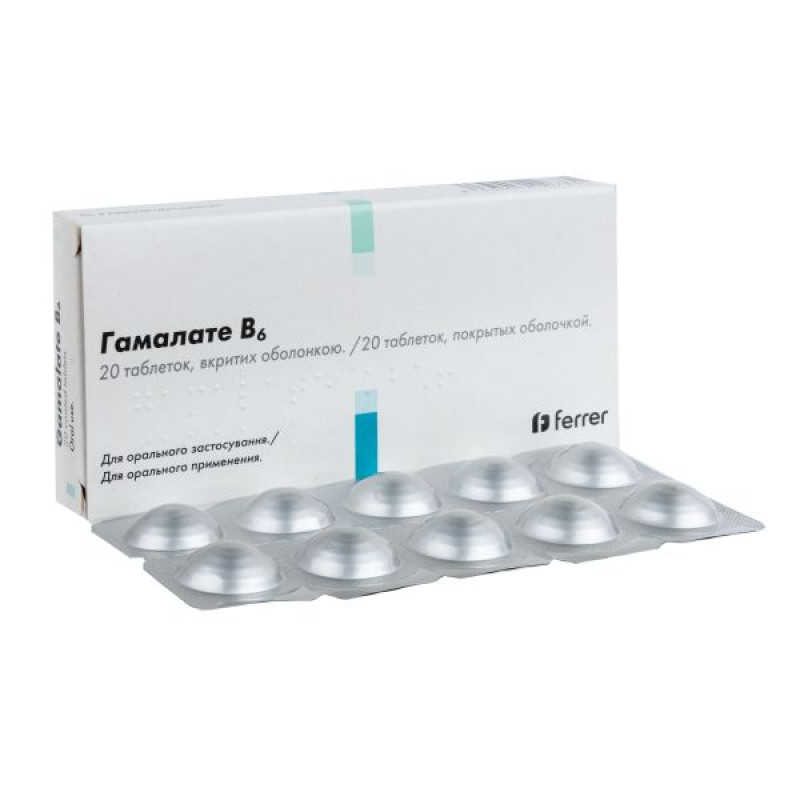
Gamalate B6 film-coated tablets blister pack No. 20
Instructions for Gamalate B6 film-coated tablets, blister pack No. 20
Composition
active ingredients: 1 film-coated tablet contains magnesium glutamate hydrobromide (anhydrous) (MGH) 75 mg; γ-aminobutyric acid (GABA) 75 mg; γ-amino-β-oxybutyric acid (GABOM) 37 mg; vitamin B6 (pyridoxine hydrochloride) 37 mg;
excipients: colloidal anhydrous silica, povidone, sodium starch glycolate (type A), magnesium stearate, talc, corn starch; coating: titanium dioxide (E 171), acacia, light magnesium carbonate, talc, acrylate copolymer, propylene glycol, sugar syrup, indigo carmine (E 132), carnauba wax.
Dosage form
Film-coated tablets.
Main physicochemical properties: sugar-coated tablets, blue in color.
Pharmacotherapeutic group
Psychostimulants and nootropics.
ATX code N06B X.
Pharmacological properties
Pharmacodynamics.
Gamalate B6 contains a combination of active substances: γ-aminobutyric acid, γ-amino-β-oxybutyric acid, vitamin B6 (pyridoxine hydrochloride), which are natural components of brain tissue, and magnesium glutamate hydrobromide (MGH). The drug has a neuroregulatory effect on brain processes, causes a mild sedative and cerebrotonic effect.
γ-Aminobutyric acid (GABA) is formed in the brain by the decarboxylation of glutamic acid. This reaction is catalyzed by the enzyme glutamine decarboxylase (GDC) and the coenzyme vitamin B6. The reaction results in the formation of γ-amino-β-oxybutyric acid (GABA). GABA has an anticonvulsant effect, which is realized through a cholinergic mechanism and improves memory and learning ability.
In addition, GABA can be converted back into its precursor (glutamic acid) through a transamination mechanism catalyzed by the enzyme GABA-ά-ketoglutarate transaminase (GABA-T), with vitamin B6 also acting as a coenzyme. Through this transamination mechanism, GABA participates in brain oxygenation.
GABA, glutamic acid, aspartic acid, which forms a substrate for the Krebs cycle in the brain.
Thus, the level of GABA in the central nervous system depends on the balance of the enzymes glutamine decarboxylase and GABA-T.
When the brain is impaired, a deficiency of inhibition is detected, which is associated with a decrease in the level of GABA - the main inhibitory neurotransmitter. Taking Gamalate B6 ensures the exogenous supply of GABA to the nervous system, and high levels of GABA, in turn, provide the following effects:
performs a neurotransmitter function and inhibits excitation processes;
participates in the transport and use of glucose in the brain;
participates in cellular respiration and oxidative phosphorylation;
promotes the combination of certain amino acids (leucine, alanine, phenylalanine) into proteins;
participates in the regulation of protein synthesis in the brain.
MGH in its structure contains glutamic acid and a magnesium compound with bromine in the form of a chelate. Thanks to the latter, MGH acts as a partial agonist of L-glutamate and causes a decrease in stimulation and a mild sedative effect. This distinguishes it from tranquilizers (benzodiazepines, barbiturates), which have a direct inhibitory effect, which is associated with a high level of side effects that are not characteristic of MGH. Studies have shown anticonvulsant activity of MGH, a positive effect on sleep disorders, neurovegetative changes and behavioral disorders in children. The sedative effect of MGH is not associated with a decrease in attention and concentration.
Pharmacokinetics.
Gamalate B6 contains four components, three of which are physiological products (GABA, GABAB and vitamin B6). Classical pharmacokinetic studies in this case are impossible due to the complexity of quantitative determination of exogenous and endogenous components. Such a number of components of the drug also makes it impossible to conduct analysis using a radioactively labeled product due to the high radiological load.
Indication
Adults as an adjunct to functional asthenia with manifestations of:
emotional lability;
impaired concentration and memory;
depression and asthenia;
low adaptability.
Contraindication
Hypersensitivity to any components of the drug.
Due to the content of γ-aminobutyric acid, the drug is contraindicated in acute renal failure.
Due to the content of pyridoxine hydrochloride, the drug is contraindicated for use in cases of gastric and duodenal ulcers in the acute stage (due to the possibility of increased acidity of gastric juice).
Interaction with other medicinal products and other types of interactions
When used together with benzodiazepine drugs (tranquilizers, anticonvulsants), as well as with sedatives (barbiturates), mutual potentiation of the effect is observed. When used together with benzodiazepine drugs, each of the medicinal substances should be prescribed in minimal or average effective doses. Pyridoxine hydrochloride is incompatible with drugs containing levodopa, since with simultaneous use, peripheral decarboxylation of levodopa is enhanced and thus its antiparkinsonian effect is reduced.
Application features
The excipients of the drug include sugar syrup, which should be taken into account by patients with diabetes mellitus. The drug should not be prescribed to patients with rare hereditary problems of fructose, glucose or galactose intolerance, malabsorption or sucrase-isomaltase deficiency.
This medicinal product contains less than 1 mmol (23 mg) sodium per dose. Caution should be exercised when administering this medicinal product to patients on a controlled sodium diet.
May cause symptoms similar to those caused by alcohol consumption due to the presence of propylene glycol.
Use during pregnancy or breastfeeding
During pregnancy or breastfeeding, the drug should be used only under the supervision of a physician and taking into account the benefit/risk ratio.
Vitamin B6 is excreted in breast milk. High concentrations of vitamin B6 may suppress milk production.
Ability to influence reaction speed when driving vehicles or other mechanisms
In some cases, some adverse reactions from the central nervous system may affect the ability to drive vehicles or operate complex mechanisms.
Method of administration and doses
Adults should take 2 tablets orally 2–3 times a day.
The duration of treatment depends on the patient's condition and the course of the disease and ranges from 1 to 6 months (determined by the doctor individually).
Children
In pediatric practice, use the drug in the form of a solution for oral administration.
Overdose
The drug has low toxicity, so intoxication is not expected.
Adverse reactions
When used in high doses, dyspeptic disorders are possible, which disappear with dose adjustment. The appearance of allergic reactions is not excluded.
On the part of the digestive tract: nausea, vomiting. The active substance pyridoxine hydrochloride can cause increased acidity of gastric juice.
Expiration date
5 years.
Storage conditions
Keep out of reach of children.
Store at a temperature not exceeding 30 °C.
Packaging
10 film-coated tablets in a blister. 2 or 6 blisters in a pack.
Vacation category
According to the recipe.
Producer
Ferrer International, S.A., Spain.
Location of the manufacturer and its business address
Legal address
Gran Via Carlos III, 94, 08028 Barcelona, Spain.
Place of production
s/Joan Buscaya, 1-9, 08173 Sant Cugat del Bayes (Barcelona), Spain.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.