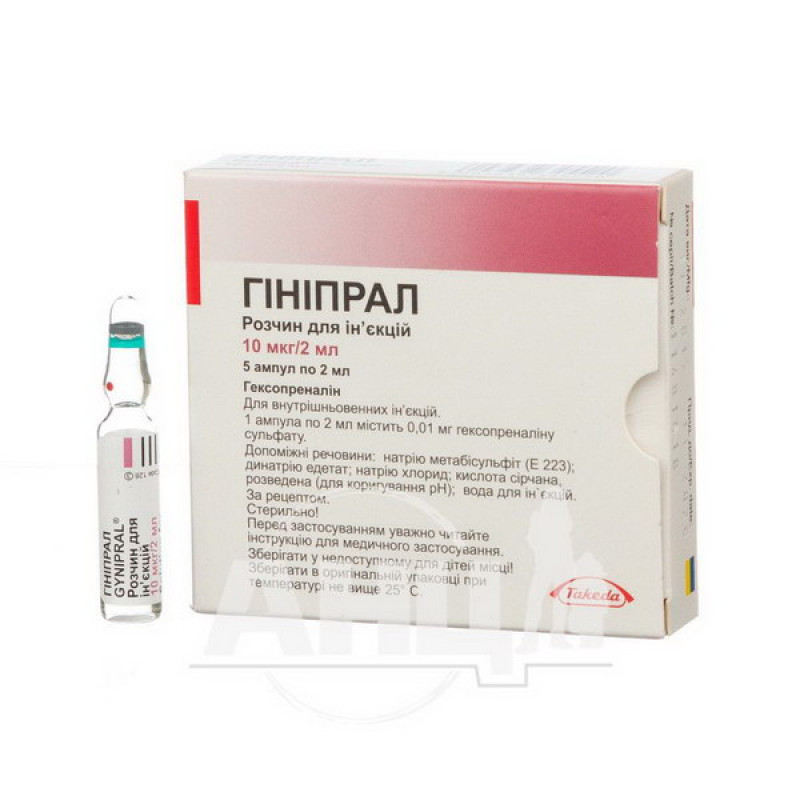
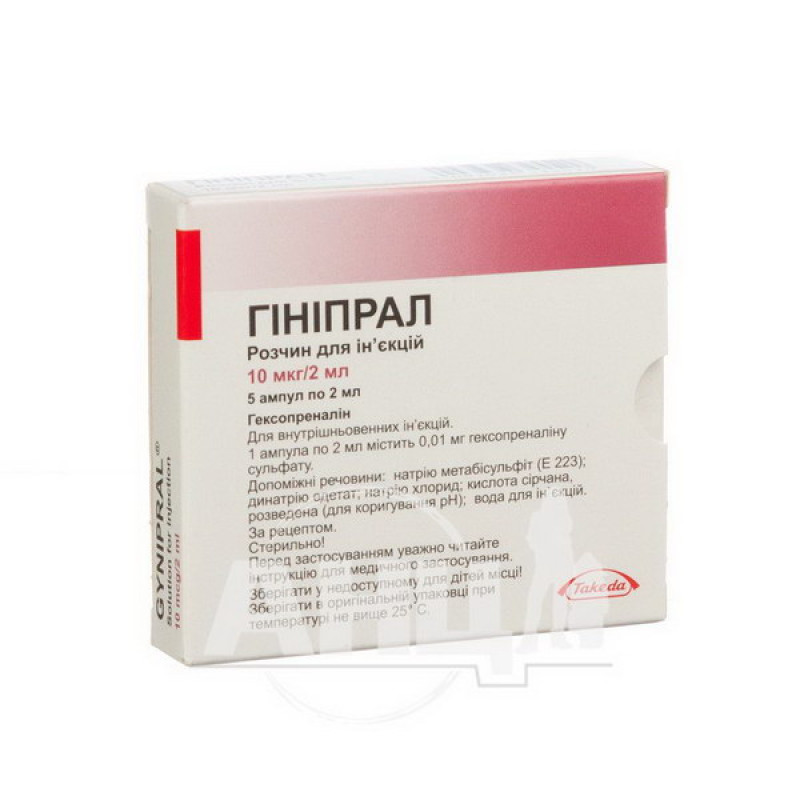
Ginipral solution for injection 10 mcg ampoule 2 ml No. 5
Ginipral injection solution is used for the following indications:
short-term treatment of uncomplicated preterm labor: suppression of uterine activity in patients with a gestational age of 22 to 37 weeks in the absence of medical or gynecological contraindications to tocolytic therapy; before turning the fetus from a transverse position; as an emergency measure in case of preterm labor before transporting the pregnant woman to the hospital.Composition
The active substance is hexoprenaline (one 2 ml ampoule contains 0.01 mg of hexoprenaline sulfate).
Excipients: sodium metabisulfite (E 223); disodium edetate; sodium chloride; sulfuric acid, diluted (for pH adjustment); water for injections.
Contraindication
hypersensitivity to hexaprenaline or to any component of the drug; presence of any disease up to 22 weeks of pregnancy; use of the drug as a tocolytic agent in patients with a history of ischemic heart disease or patients with significant risk factors for the development of ischemic heart disease; threat of miscarriage in the I and II trimesters of pregnancy; any disease of the mother or fetus in which the preservation of the pregnancy is dangerous, for example, severe toxemia, intrauterine infection, vaginal bleeding due to placenta previa, eclampsia or severe preeclampsia, placental abruption or umbilical cord compression; intrauterine fetal death, fatal congenital anomalies in history or fatal chromosomal anomalies; bronchial asthma with increased sensitivity to sulfates; diseases of the cardiovascular system, in particular tachyarrhythmia, myocarditis, mitral valve defect; hyperthyroidism; severe liver and kidney diseases; glaucoma; The use of the drug "Ginipral" is contraindicated in patients who have diseases against which the use of beta-adrenomimetics may have an undesirable effect, for example, pulmonary hypertension, diseases of the cardiovascular system (hypertrophic obstructive cardiomyopathy or obstruction of blood flow from the left ventricle, for example, aortic stenosis).Method of application
For intravenous injection or infusion.
The indicated dosage can only be used as a guideline, since tocolysis requires an individual approach to the patient.
Ginipral therapy should only be initiated by obstetricians/physicians experienced in the use of tocolytic agents. Treatment should only be administered in properly equipped facilities to ensure continuous monitoring of the health of the mother and fetus.
The drug "Ginipral" should be used as early as possible after diagnosing premature labor and excluding any contraindications to the use of hexoprenaline.
During therapy with the drug, it is necessary to properly assess the state of the cardiovascular system using continuous ECG monitoring.
Dosage
Before turning the fetus from the transverse position and when used as an emergency measure in premature labor before transporting the pregnant woman to the hospital. The recommended dose is 10 mcg (one ampoule of 2 ml) of the drug "Ginipral", diluted in 10 ml of 0.9% sodium chloride solution or 5% glucose solution, administered intravenously over 5-10 minutes. If necessary, continue treatment with the drug "Ginipral", concentrate for the preparation of a solution for infusions.
Short-term treatment of premature labor in the presence of shortening and / or opening of the cervix. At the beginning of treatment, start with a jet dose of 10 mcg (one ampoule of 2 ml) followed by infusion of the drug "Ginipral" at a rate of 0.3 mcg / min. As an alternative treatment, it is possible to use only infusions of the drug "Ginipral" at a rate of 0.3 mcg / min without prior jet injection of the drug. Administer intravenously drip (when calculating the rate of administration using conventional infusion systems, take into account that 20 drops = 1 ml). Dissolve the required number of ampoules of concentrate for infusions in 500 ml of 0.9% sodium chloride solution or 5% glucose solution.
Short-term treatment of premature labor without shortening or opening of the cervix. Recommended dose: continuous infusion of 0.075 mcg / minute. Administer intravenously by drip (when calculating the rate of administration using conventional infusion systems, take into account that 20 drops = 1 ml). Dissolve the required number of ampoules of concentrate for infusion in 500 ml of 0.9% sodium chloride solution or 5% glucose solution.
Duration of treatment
The duration of tocolytic treatment depends on the existing risk of miscarriage (tendency to shorten the interval between contractions, the condition of the cervix) and the severity of side effects (e.g. increased heart rate). Side effects should be minimized as much as possible.
The duration of treatment should not exceed 48 hours, as research data indicate that tocolytic therapy can delay labor for up to 48 hours. In randomized controlled trials, no statistically significant effect of Ginipral treatment on perinatal mortality or morbidity was observed. Delaying labor with Ginipral can be used to implement other measures that significantly improve perinatal health.
Application features
Children
The drug is not intended for use in children.
The drug has no or negligible effect on the reaction rate when driving vehicles or using other mechanisms.
Overdose
Symptoms of overdose may include: a significant increase in the mother's heart rate, tremors, palpitations, headache, sweating.
Usually, to eliminate these symptoms, it is enough to reduce the dosage of the drug. To eliminate severe symptoms of overdose, the use of non-selective beta-blockers is recommended, which competitively inhibit the action of the drug "Ginipral".
Side effects
The most common side effects of the drug "Ginipral" are associated with the pharmacological properties of beta-adrenomimetics. In order to eliminate or reduce the occurrence of undesirable effects, careful monitoring of hemodynamic parameters, such as blood pressure, heart rate and appropriate adjustment of the drug dose are carried out. These effects usually disappear after discontinuation of the drug.
Metabolic disorders: often (≥ 1: 100, <1:10) - hypokalemia.
Nervous system disorders: very common (≥ 1:10) - tremor.
From the cardiovascular system: very often (≥ 1:10) - tachycardia; often (≥ 1:100, <1:10) - palpitations, decreased diastolic pressure, arterial hypotension.
Skin and subcutaneous tissue disorders: common (≥ 1:100, <1:10) - sweating.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 °C, out of the reach of children.
Shelf life - 3 years.
Reconstituted solution: Chemical and physical in-use stability of the reconstituted solution (0.9% sodium chloride solution or 5% glucose solution) has been demonstrated for 24 hours at 15-25°C. From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.