Glenspray Active nasal spray, dosed suspension, bottle of 150 doses with a spray pump
Instructions for Glenspray Active nasal spray, dosed suspension, bottle of 150 doses with a spray pump
Composition
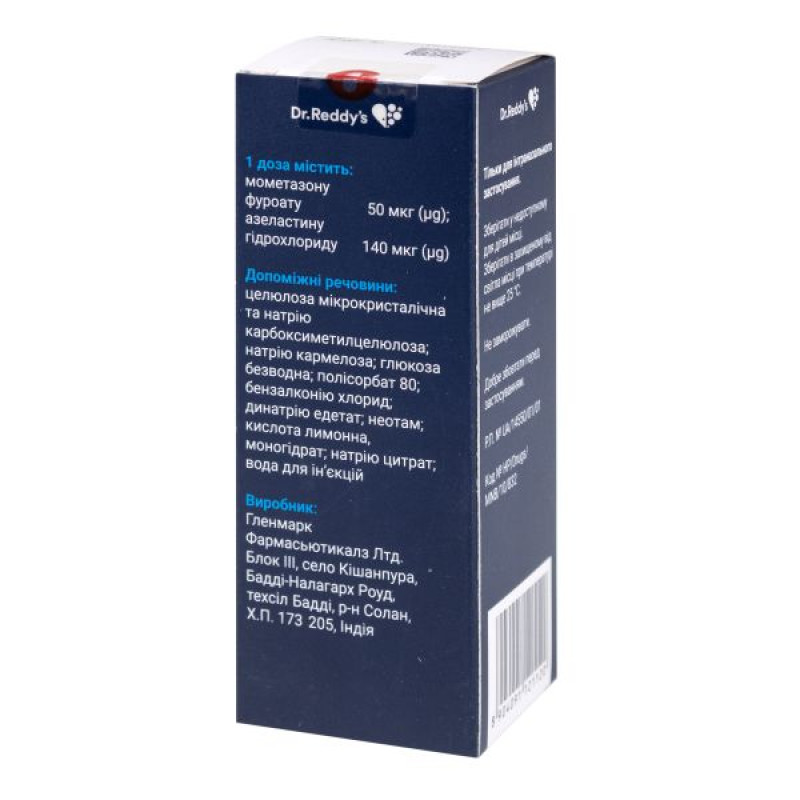
active ingredients: mometasone furoate and azelastine hydrochloride;
1 dose contains mometasone furoate 50 mcg and azelastine hydrochloride 140 mcg;
Excipients: microcrystalline cellulose and sodium carboxymethylcellulose; carmellose sodium; anhydrous glucose; polysorbate 80; benzalkonium chloride; disodium edetate; neotame; citric acid monohydrate; sodium citrate; water for injections.
Dosage form
Nasal spray, metered dose, suspension.
Main physicochemical properties: white or almost white suspension.
Pharmacotherapeutic group
Anti-edematous and other drugs for local use in diseases of the nasal cavity. Corticosteroids. ATX code R01A D.
Pharmacological properties
Pharmacodynamics
A combined decongestant for topical use containing mometasone furoate and azelastine hydrochloride.
Azelastine hydrochloride is a derivative of phthalazinone. It has a prolonged antiallergic effect. It has pronounced selective properties of an antagonist of histamine H1 receptors. Azelastine inhibits the synthesis or release of chemical mediators involved in the development of early and late stages of allergic reactions, such as leukotrienes, histamine, PAF inhibitors and serotonin. The main metabolite of azelastine is desmethylazelastine, which is also an antagonist of histamine H1 receptors.
Mometasone furoate is a synthetic corticosteroid for topical use that has a pronounced anti-inflammatory effect. The exact mechanism of action of corticosteroids in allergic rhinitis is still unknown. Corticosteroids demonstrate a wide range of effects on various cells, namely heparinocytes, eosinophils, neutrophils, macrophages, lymphocytes, as well as on inflammatory mediators (histamine, eicosanoids, leukotrienes and cytokines). The mechanism of anti-inflammatory and anti-allergic action of mometasone furoate is mainly associated with its ability to inhibit the release of mediators of allergic reactions.
Studies have shown that mometasone furoate in the form of a nasal spray 50 mcg/dose when applied topically reduces the level of some mediators of the early and late phase of the allergic reaction, reduces (compared to placebo) the level of histamine and eosinophil cationic protein, and reduces (compared to baseline) the number of eosinophils, neutrophils, and epithelial cell adhesion proteins.
Pharmacokinetics
A separate pharmacokinetic study of a nasal spray containing azelastine hydrochloride and mometasone furoate (140 mcg/50 mcg) has not been conducted.
Absorption
After intranasal administration of 2 actuations in each nostril of azelastine nasal spray (total dose 548 mcg), the mean peak plasma concentration (Cmax) of azelastine is 200 pg/mL, the mean systemic exposure (AUC) is 5122 pg x h/mL, and the mean time (Tmax) to reach Cmax is 3 hours. The systemic bioavailability of azelastine hydrochloride after intranasal administration is approximately 40%.
Mometasone furoate suspension when administered as a nasal spray has very low bioavailability (<1%), as demonstrated by a sensitive quantitative assay with a lower limit of quantification (LOQ) of 0.25 pg/mL.
Distribution
With intravenous and oral administration, the equilibrium volume of distribution of azelastine is 14.5 l/kg. In vitro, the plasma protein binding of azelastine and its active metabolite desmethylazelastine is 88% and 97%, respectively.
In vitro, the plasma protein binding of mometasone furoate is 98 to 99% over a concentration range of 5 to 500 ng/mL.
Metabolism
Azelastine is metabolized by oxidation by the cytochrome P450 enzyme system to the main active metabolite, desmethylazelastine. Specific P450 isoforms responsible for the biotransformation of azelastine have not been identified. After a single intranasal application of azelastine nasal spray (total dose of 548 μg), the mean Cmax of desmethylazelastine is 23 pg/ml, AUC is 2131 pg × h/ml, and the mean Tmax is 24 hours. After reaching steady-state concentrations of azelastine with intranasal administration, plasma concentrations of desmethylazelastine were in the range of 20–50% of those of azelastine.
Studies have shown that a portion of the ingested and absorbed dose of mometasone furoate undergoes active metabolism. No major metabolites were detected in plasma. After in vitro incubation, one of the minor metabolites formed was 6β-hydroxymometasone furoate. In human liver microsomes, metabolite formation is regulated by cytochrome P450 3A4 (CYP3A4).
Breeding
After intranasal administration of azelastine nasal spray, the half-life of azelastine was 22 hours and that of desmethyl azelastine was 52 hours. Approximately 75% of an oral dose of radiolabeled azelastine hydrochloride was excreted in the feces, with less than 10% as unchanged azelastine.
After intravenous administration, the half-life of mometasone furoate is 5.8 hours. It is excreted from the body as metabolites mainly with bile and to a limited extent with urine.
Pharmacokinetics in certain patient groups.
After oral administration of azelastine, hepatic insufficiency did not affect pharmacokinetic parameters.
Administration of a single inhaled dose of 400 mcg of mometasone furoate to patients with mild, moderate, and severe hepatic impairment resulted in peak plasma concentrations of mometasone furoate (50 to 105 pg/mL) in only 1 or 2 patients from each group. The observed peak plasma concentrations increased with increasing severity of hepatic impairment.
Kidney failure
Studies of single oral doses of azelastine in patients with renal insufficiency (creatinine clearance <50 ml/min) showed a 70-75% increase in Cmax and AUC compared to healthy volunteers. The time to peak concentration was unchanged.
The effect of renal insufficiency on the pharmacokinetics of mometasone furoate has not been studied.
Age
Age did not affect the pharmacokinetic parameters of azelastine when administered orally. The pharmacokinetics of mometasone furoate have not been studied in the pediatric population.
Sex
Gender did not affect the pharmacokinetic parameters of azelastine administered orally. The effect of gender on the pharmacokinetics of mometasone furoate has not been studied.
Indication
Seasonal allergic rhinitis.
Perennial allergic rhinitis.
Contraindication
Hypersensitivity to azelastine hydrochloride, mometasone furoate or to any other component of the drug.
Untreated local infection of the nasal mucosa.
Nasal trauma or recent nasal surgery.
Interaction with other medicinal products and other types of interactions
Specific drug interaction studies of the fixed-dose combination of azelastine hydrochloride and mometasone furoate nasal spray have not been conducted. The following are data on the individual components that make up the drug. Since mometasone and azelastine act on different types of receptors, drug interactions are not expected with this combination.
Azelastine hydrochloride
Central nervous system depressants
Concomitant use of azelastine with alcohol or other central nervous system depressants may result in decreased concentration and deterioration of central nervous system function.
Erythromycin and ketoconazole
ECG evaluation of patients receiving oral azelastine hydrochloride and erythromycin or ketoconazole concomitantly showed no clinically significant effect on the QT interval (or QTc value). Oral erythromycin 500 mg 3 times daily for 7 days had no effect on the pharmacokinetics of azelastine or QTc value. Ketoconazole 200 mg 2 times daily for 7 days interfered with the measurement of plasma azelastine concentrations by analytical high-performance liquid chromatography (HPLC); however, no effect on QTc value was observed.
Cimetidine
Cimetidine at a dose of 400 mg twice daily when administered orally with azelastine hydrochloride at a dose of 4 mg twice daily increases the mean Cmax and AUC of the latter by approximately 65%.
Mometasone furoate
Cytochrome P450 3A4 inhibitors
Ketoconazole
Mometasone furoate is primarily metabolized in the liver. In vitro studies have confirmed the primary role of cytochrome CYP3A4 in the metabolism of this compound. Concomitant use with ketoconazole, a potent inhibitor of CYP3A4, may increase plasma concentrations of mometasone furoate.
Ritonavir
Ritonavir, as a protease inhibitor, may inhibit the metabolism of mometasone furoate when used concomitantly, leading to increased systemic exposure to the latter and an increased risk of side effects.
Loratadine
Clinical drug interaction studies have been conducted with loratadine. No interactions were observed.
Application features
Azelastine hydrochloride
In clinical trials, drowsiness has been reported in some patients using azelastine hydrochloride nasal spray. During treatment with the drug, patients should refrain from work that requires increased concentration and speed of reactions.
Concomitant use of azelastine nasal spray with alcohol or other central nervous system depressants should be avoided due to possible suppression of central nervous system function.
Mometasone furoate
Local effects on the nose
Nosebleed
In clinical trials, epistaxis was observed more frequently in patients with allergic rhinitis who received mometasone furoate nasal spray than in those who received placebo.
Fungal infection
In clinical studies with mometasone furoate nasal spray 50 mcg/dose, isolated cases of localized fungal infection of the nose and throat (Candida albicans) were observed. If a fungal infection occurs, the drug should be discontinued and appropriate treatment should be initiated.
Isolated cases of nasal septal perforation have been reported following the use of intranasal corticosteroids. As with any long-term treatment, patients using mometasone furoate nasal spray for several months or longer should be periodically examined for possible changes in the nasal mucosa.
Impaired wound healing
Due to the inhibitory effect of corticosteroids on wound healing, patients who have recently had nasal septal ulcers, nasal surgery, or nasal trauma should not use nasal corticosteroids until they have fully recovered.
Vision impairment
Visual disturbances may occur with systemic and topical corticosteroids (including intranasal, inhaled, and intraocular administration). If symptoms such as blurred vision or other visual disturbances occur, the patient should be evaluated by an ophthalmologist to evaluate possible causes of visual disturbances, which may include cataracts, glaucoma, or rare conditions such as central serous chorioretinopathy, which have been reported following the use of systemic and topical corticosteroids.
Hypersensitivity reactions
Hypersensitivity reactions, including wheezing, may occur after intranasal administration of mometasone furoate. In such cases, therapy with the drug should be discontinued.
Immunosuppression
Patients treated with drugs that suppress the immune system are more susceptible to infections than healthy people. For example, chickenpox and measles are more severe in immunocompromised children and adults who are taking corticosteroids. Such patients require special attention and the use of antiviral drugs or vaccination. The effect of the dose, route, and duration of corticosteroid use on the risk of developing generalized infection is not yet known.
Mometasone furoate nasal spray should be used with caution or not at all in patients with active or latent tuberculosis infection of the respiratory tract, as well as in untreated fungal, bacterial, systemic viral infections, or herpes simplex infection with ocular involvement.
Effect on the hypothalamic-pituitary-adrenal system
Hyperadrenocorticism and adrenal suppression
With prolonged use of intranasal corticosteroids, especially at high doses, systemic effects such as hyperadrenocorticism and adrenal suppression may occur. It is important that when a therapeutic effect is achieved, the dose of intranasal corticosteroids be reduced to the minimum effective dose that controls the course of the disease, in order to avoid the development of systemic side effects.
If signs of undesirable systemic effects appear, treatment with mometasone furoate nasal spray should be gradually discontinued.
Impact on growth dynamics in children
Corticosteroids may cause growth retardation in children. It is recommended that growth be monitored regularly in children treated with intranasal corticosteroids for long periods of time.
Glenspray Active contains benzalkonium chloride, which may cause irritation of the nasal mucosa and bronchospasm.
It should be remembered that corticosteroids have a particularly potent diabetogenic effect and can lead to increased hepatic gluconeogenesis and decreased glucose uptake by peripheral tissues and hyperglycemia.
Use in elderly patients.
There are no clinical data on the safety of the drug in elderly patients.
Ability to influence reaction speed when driving vehicles or other mechanisms
No special studies have been conducted on the effect of the drug on the ability to drive vehicles and other mechanisms.
Since drowsiness has been reported in some patients who used azelastine hydrochloride nasal spray in clinical studies, patients should refrain from performing tasks that require increased concentration and speed of reactions during treatment with the drug.
Use during pregnancy or breastfeeding
No specific studies of the drug's effects during pregnancy or breastfeeding have been conducted.
Use during pregnancy.
Azelastine hydrochloride. There are no adequate and well-controlled clinical trials in pregnant women. Azelastine hydrochloride has been shown to be embryotoxic in animal studies.
Mometasone furoate: There are no adequate and well-controlled clinical trials in pregnant women.
It should be noted that due to the natural increase in corticosteroid production during pregnancy, most women require lower doses of exogenous corticosteroids, and many do not require corticosteroid treatment during pregnancy.
Use during breastfeeding.
Azelastine hydrochloride: It is not known whether azelastine hydrochloride is excreted in human milk.
Mometasone furoate: It is not known whether mometasone furoate is excreted in human milk.
Method of administration and doses
The drug is intended for intranasal use only.
Patients with renal impairment. No dose adjustment is required in patients with mild renal impairment (creatinine clearance > 79 ml/min). In patients with moderate and severe renal impairment (creatinine clearance < 79 ml/min – > 10 ml/min), the drug should be used with caution and under close medical supervision.
Patients with hepatic insufficiency: No dose adjustment is required.
Using a nasal spray
Before each use, shake the bottle gently for 5 seconds. Then remove the protective cap. Before using the bottle for the first time, press the dosing device 6 times in a row. If the nasal spray has not been used for more than 7 days, press the dosing pump-sprayer again 6 times in a row before use.
Before each use, the nose should be thoroughly cleaned of mucus. After cleaning the nose, the suspension is injected into each nostril, while the head should be kept slightly tilted downwards. After use, the spray tip should be wiped and covered with a protective cap.
Children
There is no sufficient clinical experience with the drug in children under 12 years of age, so it should not be used in patients in this age category.
Overdose
There are no reports of overdose with Glenspray Active.
In case of acute overdose, central nervous system disorders (drowsiness, dizziness) are possible. Oral single administration of azelastine hydrochloride in doses up to 16 mg during clinical studies did not lead to an increase in the frequency of serious adverse effects. Treatment is symptomatic. Antidote is unknown.
There are no reports of acute or chronic overdose with mometasone furoate nasal spray. Single intranasal doses of up to 4 mg and oral inhalation doses of up to 8 mg have been reported in volunteers without any adverse effects.
Chronic overdose with any corticosteroid may lead to symptoms of hypercorticism.
Adverse reactions
In a clinical trial, adverse events associated with the use of azelastine hydrochloride/mometasone furoate nasal spray were reported in 11 of 282 patients enrolled in the study. A total of 18 adverse events were reported with the combination of azelastine and mometasone. The most common adverse events were headache and dysgeusia. Other adverse events included somnolence, lethargy, nausea, dyspepsia, and sneezing. Most adverse events were mild in severity, and no serious adverse events were reported during the study.
Below are the adverse reactions that have been observed with the use of individual components of the drug.
Azelastine hydrochloride nasal spray
Common (1-10%): A specific bitter taste may appear after using the spray (most often due to incorrect application, namely when the head is tilted back too far during injection), which in some cases may lead to nausea.
Uncommon (0.1-1%): Temporary irritation of the inflamed nasal mucosa may occur with symptoms such as burning, itching, sneezing and nosebleeds.
In very rare cases (< 0.01%) hypersensitivity reactions (rash, itching, urticaria) have been reported.
Post-registration experience
Adverse reactions identified during post-marketing use of azelastine nasal spray: abdominal pain, burning in the nose, nausea, sweet taste, throat irritation, anaphylactoid reactions, irritation at the application site, atrial fibrillation, visual disturbances (blurred vision, cataract, glaucoma, central serous chorioretinopathy (see section "Special warnings and precautions for use")), chest pain, confusion, dizziness, shortness of breath, facial swelling, hypertension, involuntary muscle contractions, nervousness, palpitations, paresthesias, parosmia, paroxysmal sneezing, itching, rash, impaired or loss of smell and/or taste, tachycardia, immunological tolerance, urinary retention, xerophthalmia. Because these reactions concern a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the use of the drug.
Mometasone furoate nasal spray
Respiratory, thoracic and mediastinal disorders: epistaxis, pharyngitis, burning or irritation in the nose, nasal ulcers - common (1 - 10%).
General disorders and local reactions: headache - common (1 - 10%).
Nosebleeds were mostly mild and did not require medical intervention.
In children, the most common symptoms were nosebleeds, headache, irritation of the nasal mucosa, and sneezing.
Systemic side effects during treatment with nasal corticosteroids may occur when high doses are used for a long time.
Post-registration experience
Adverse reactions identified during post-marketing use of mometasone furoate nasal spray: burning and irritation in the nose, anaphylaxis and angioedema, taste and smell disorders, and nasal septal perforation.
The use of Glenspray Active may lead to the development of kidney and urinary system disorders (hematuria).
Corticosteroids stimulate acid and pepsin secretion, which can lead to gastritis and hyperchlorhydria.
Expiration date
2 years.
Storage conditions
Store in a dark place at a temperature not exceeding 25 °C. Do not freeze. Keep out of the reach of children.
Packaging
150 doses in a polyethylene bottle. 1 bottle with a dosing pump-sprayer, closed with a cap, in a cardboard box.
Vacation category
According to the recipe.
Producer
Glenmark Pharmaceuticals Ltd.
Location of the manufacturer and its business address
Block III, Village Kishanpura, Baddi-Nalagarh Road, Tehsil Baddi, District Solan, H.P. 173 205, India.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.