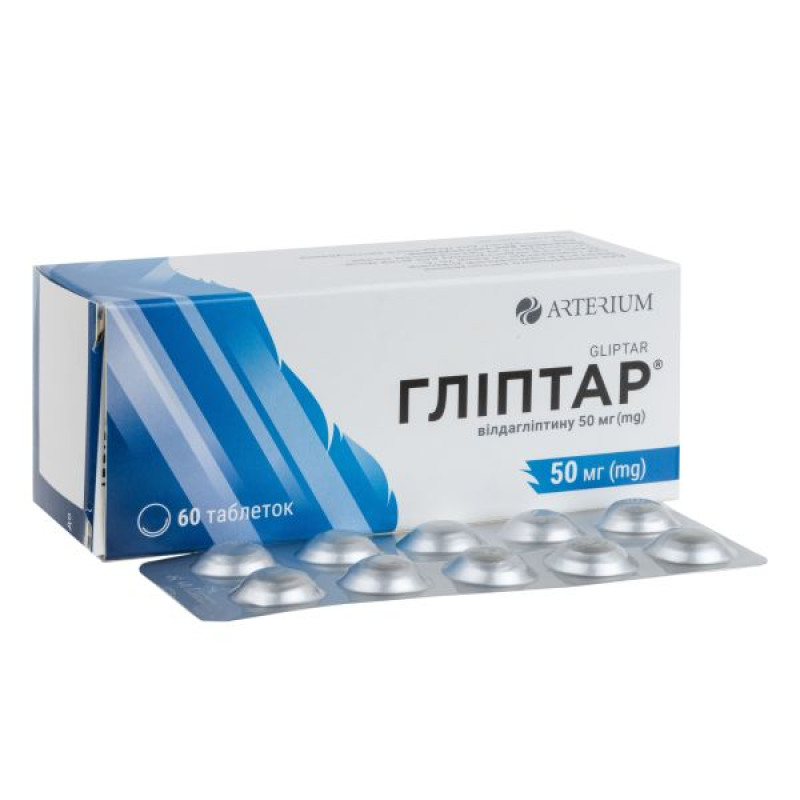
Glyptar tablets 50 mg blister No. 60

Instructions for Glyptar tablets 50 mg blister No. 60
Composition
active ingredient: vildagliptin;
1 tablet contains vildagliptin 50 mg;
excipients: microcrystalline cellulose, anhydrous lactose, sodium starch glycolate (type A), magnesium stearate.
Dosage form
Pills.
Main physicochemical properties: tablets from white to light yellow in color, round in shape with a flat surface, with a bevel.
Pharmacotherapeutic group
Hypoglycemic drugs, except insulins. Dipeptidyl peptidase-4 inhibitors. Vildagliptin.
ATX code A10B H02.
Pharmacological properties
Pharmacodynamics: Vildagliptin belongs to the class of substances that enhance the function of beta cells of the pancreatic islet apparatus, it is a potent and selective inhibitor of dipeptidyl peptidase-4 (DPP-4).
Vildagliptin administration results in rapid and complete inhibition of DPP-4 activity. Inhibition of DPP-4 by vildagliptin results in increased endogenous levels of the incretin hormones GLP-1 (glucagon-like peptide 1) and GIP (glucose-dependent insulinotropic peptide) during fasting and after meals.
By increasing endogenous levels of these incretin hormones, vildagliptin improves beta-cell sensitivity to glucose, leading to increased glucose-dependent insulin secretion. Treatment of patients with type 2 diabetes with the drug at doses of 50 to 100 mg per day significantly improved markers of beta-cell function, including HOMA-β (homeostatic model of β-cell function assessment), proinsulin to insulin ratio, and beta-cell sensitivity in a multiple meal tolerance test. In non-diabetic patients (with normal blood glucose levels), vildagliptin does not stimulate insulin secretion or lower glucose levels.
By increasing endogenous GLP-1 levels, vildagliptin also increases the sensitivity of alpha cells to glucose, leading to increased glucose-dependent glucagon secretion. The significant increase in the insulin/glucagon ratio during hyperglycemia caused by increased incretin hormone levels leads to a decrease in fasting and postprandial glucose production, which causes a decrease in glycemia.
The known effect of increased GLP-1 levels, which is to prolong gastric emptying, is not observed during treatment with vildagliptin.
Pharmacokinetics.
Absorption. After oral administration on an empty stomach, vildagliptin is rapidly absorbed, with Cmax observed after 1.7 hours. Simultaneous administration with food slightly delays the time to reach Cmax in blood plasma - up to 2.5 hours, but does not affect the total exposure (AUC). The use of vildagliptin with food leads to a decrease in the maximum concentration Cmax (19%). Despite this, the magnitude of the change is not clinically significant, so Gliptar® can be taken regardless of food intake. Absolute bioavailability is 85%.
Distribution. The plasma protein binding of vildagliptin is low (9.3%); vildagliptin is distributed evenly between plasma and erythrocytes. The mean volume of distribution of vildagliptin at plateau after intravenous administration (Vss) is 71 liters, indicating extravascular distribution.
Metabolism. Metabolism is the major route of elimination of vildagliptin in humans, accounting for 69% of the administered dose. The major metabolite, LAY151, is pharmacologically inactive and is a hydrolysis product of the cyanide moiety, accounting for 57% of the dose, followed by glucuronide (BQS867) and amide hydrolysis (4% of the dose). Data from an in vitro study in human kidney microsomes suggest that the kidney may be one of the major organs contributing to the hydrolysis of vildagliptin to its major inactive metabolite, LAY151. DPP-4 is partially involved in the hydrolysis of vildagliptin, which was confirmed by an in vivo study in DPP-4-deficient rats.
Vildagliptin is not metabolised by cytochrome P450 enzymes to a detectable extent. Therefore, concomitant administration of medicinal products such as CYP450 inhibitors and/or inducers is not expected to affect the metabolic clearance of vildagliptin. In vitro studies have shown that vildagliptin does not inhibit or induce cytochrome P450 enzymes. Therefore, vildagliptin is unlikely to affect the metabolic clearance of concomitantly administered medicinal products metabolised by CYP 1A2, CYP 2C8, CYP 2C9, CYP 2C19, CYP 2D6, CYP 2E1 or CYP 3A4/5.
Elimination. After oral administration of [14C]-vildagliptin, approximately 85% of the dose is excreted in the urine and 15% in the feces. Renal excretion of unchanged vildagliptin accounts for 23% of the oral dose. After intravenous administration to healthy volunteers, the total plasma and renal clearance of vildagliptin are 41 l/h and 13 l/h, respectively. The mean elimination half-life after intravenous administration is approximately 2 hours. The elimination half-life after oral administration is approximately 3 hours.
Linearity/non-linearity: Vildagliptin maximum plasma concentration (Cmax) and area under the plasma concentration-time curve (AUC) increase in an approximately dose-proportional manner over the entire therapeutic dose range.
Gender: No differences in pharmacokinetics were observed in healthy male and female volunteers of different ages and body mass index (BMI). DPP-4 inhibition by vildagliptin is independent of gender.
Liver disease. The effect of hepatic impairment on the pharmacokinetics of vildagliptin was studied in patients with mild, moderate and severe hepatic impairment according to the Child-Pugh classification (6 for mild to 12 for severe) compared with patients with normal hepatic function. Vildagliptin exposure after a single dose in patients with mild and moderate hepatic impairment was reduced (by 20% and 8%, respectively), while vildagliptin exposure in patients with severe impairment was increased by 22%. The maximum change (increase or decrease) in vildagliptin exposure was approximately 30%, which is not considered clinically relevant. There was no relationship between the severity of hepatic impairment and changes in vildagliptin exposure.
Renal disease: An open-label, multiple-dose study was conducted to evaluate the pharmacokinetics of the lowest therapeutic doses of vildagliptin (50 mg once daily) in patients with varying degrees of chronic renal impairment, as defined by creatinine clearance (mild renal impairment - 50 to < 80 mL/min, moderate renal impairment - 30 to < 50 mL/min, and severe renal impairment - < 30 mL/min), compared with a control group of study participants with normal renal function.
In patients with mild, moderate and severe renal impairment, the AUC of vildagliptin was increased compared to patients with normal renal function. The AUC of the metabolites LAY151 and BQS867 increased on average by approximately 1.5-, 3- and 7-fold in patients with mild, moderate and severe renal impairment, respectively. Some data in patients with end-stage renal disease (ESRD) suggest that vildagliptin exposure is similar to that in patients with severe renal impairment. LAY151 concentrations were approximately 2- to 3-fold higher than in patients with severe renal impairment.
Vildagliptin was removed from the body by hemodialysis to a limited extent (3% during 3–4 hours of hemodialysis, which was started 4 hours after drug administration).
Elderly: In healthy subjects (aged 70 years and older), total exposure to vildagliptin (100 mg once daily) was increased by 32% and maximum plasma concentration by 18% compared to younger healthy subjects (aged 18 to 40 years).
These changes, however, are not considered clinically significant. DPP-4 inhibition by vildagliptin is independent of age in the age groups studied.
Race: Limited data suggest that race does not have a significant effect on the pharmacokinetics of vildagliptin.
Indication
Treatment of adult patients with type II diabetes mellitus.
As monotherapy:
for patients in whom diet and exercise alone do not provide sufficient control, as well as for patients in whom the use of metformin is considered inappropriate due to contraindications or intolerance.
As part of dual oral therapy in combination with:
metformin for patients with inadequate glycemic control despite the use of the maximum tolerated dose during metformin monotherapy;
sulfonylurea for patients with inadequate glycemic control despite the use of the maximum tolerated dose of sulfonylurea, and for patients for whom the use of metformin is considered inappropriate due to contraindications or intolerance;
thiazolidinedione for patients with inadequate glycemic control for whom the use of a thiazolidinedione is considered appropriate.
As part of triple oral therapy in combination with:
sulfonylurea and metformin when diet and exercise together with dual therapy with these drugs do not provide adequate glycemic control.
In combination with insulin (with or without metformin) when diet and exercise together with a stable dose of insulin do not provide adequate glycemic control.
Contraindication
Known hypersensitivity to vildagliptin or to any of the excipients.
Interaction with other medicinal products and other types of interactions
Vildagliptin has a low potential for drug interactions. Since vildagliptin is not a substrate of cytochrome P450 (CYP) enzymes and is not an inhibitor or inducer of CYP 450 enzymes, its interactions with other drugs that are substrates, inhibitors or inducers of these enzymes are unlikely.
Combination with pioglitazone, metformin, and glyburide
The results of studies conducted with these oral antidiabetic agents did not show a clinically significant pharmacokinetic interaction.
Digoxin (Pgp substrate), warfarin (CYP2C9 substrate)
Clinical studies conducted in healthy volunteers did not show any clinically significant pharmacokinetic interaction. However, this has not been established in the target population.
Drug interaction studies in healthy volunteers have been conducted with amlodipine, ramipril, valsartan and simvastatin. No clinically significant pharmacokinetic interactions were observed in these studies when these drugs were co-administered with vildagliptin.
Combination with ACE inhibitors.
There may be an increased risk of angioedema in patients taking concomitant angiotensin-converting enzyme [ACE] inhibitors (see section 4.8).
As with other oral antidiabetic drugs, certain active substances, including thiazides, corticosteroids, thyroid hormone preparations and sympathomimetics, may reduce the hypoglycaemic effect of vildagliptin.
Application features
During the treatment of diabetes, it is always necessary to follow a low-calorie diet and control weight. Regular physical activity is important. The recommendations are appropriate not only for the primary therapy of diabetes, but also as a supplement to drug treatment.
Gliptar® is not a substitute for insulin in insulin-dependent patients. The drug should not be used to treat patients with type 1 diabetes or diabetic ketoacidosis.
Kidney dysfunction
Experience in the treatment of patients with moderate or severe renal impairment, as well as patients with end-stage renal disease (ESRD) on hemodialysis is limited. Therefore, the use of Gliptar® is not recommended in these patient groups.
Liver dysfunction
Gliptar® is not recommended for use in patients with impaired liver function, including patients whose ALT (alanine aminotransferase) or AST (aspartate aminotransferase) levels were more than 2.5 times the upper limit of normal before treatment.
Creatinine clearance should be checked before starting treatment and regularly during treatment.
Monitoring liver enzyme levels
Liver function disorders (including hepatitis) have been reported rarely. In such cases, the course of the complication in patients was mostly asymptomatic, without clinical consequences, and liver function tests (LFTs) returned to normal after discontinuation of treatment. Before starting treatment with Gliptar®, LFTs should be performed to determine the patient's baseline values. LFTs should be monitored during treatment with the drug at intervals of once every three to four months during the first year of treatment, and periodically thereafter.
Patients who have experienced elevated transaminase levels should have their liver function tested again to confirm the results and should be monitored frequently with liver function tests until the abnormal levels return to normal. If ALT or AST levels increase to 3 or more times the upper limit of normal, it is recommended that Gliptar® be discontinued. Patients who develop jaundice or other signs of liver dysfunction should discontinue Gliptar®. Vildagliptin should not be restarted after discontinuation of treatment and TFP has returned to normal.
Heart failure
A clinical study of vildagliptin in patients with heart failure in NYHA functional classes I–III (the functional classification of chronic heart failure of the New York Heart Association) showed that treatment with vildagliptin was not associated with changes in left ventricular function or worsening of existing congestive heart failure. Clinical experience in patients with NYHA functional class III is still limited and the results are inconclusive.
There is no experience of using vildagliptin in clinical trials in patients with NYHA functional class IV heart failure, therefore the drug is not recommended for use in these patients.
Skin disorders
In nonclinical toxicology studies, skin lesions, including blistering and ulceration of the extremities in monkeys, were reported. Although no increased incidence of skin lesions was observed in clinical studies, experience with skin complications in patients with diabetes is limited.
In addition, cases of bullous and exfoliative skin lesions have been reported in the post-marketing period.
Therefore, as per standard care for patients with diabetes, monitoring for skin abnormalities such as blistering or ulceration is recommended.
Pancreatitis
The use of the drug Gliptar® is associated with the risk of developing acute pancreatitis. Patients should be informed about the characteristic symptoms of acute pancreatitis. Caution should be exercised in patients with a history of acute pancreatitis.
If pancreatitis is suspected, use of Gliptar® should not be continued. If the diagnosis of acute pancreatitis is confirmed, use of Gliptar® should not be resumed.
The use of sulfonylureas is known to cause hypoglycemia. Patients receiving Gliptar® in combination with a sulfonylurea may be at increased risk of hypoglycemia. Therefore, lower doses of the sulfonylurea may be used to reduce the risk of hypoglycemia.
Myopathy/rhabdomyolysis
Myopathy, manifested by muscle pain, weakness, or tenderness, in association with markedly elevated creatine kinase [CK] (>10 times the upper limit of normal), has been reported with vildagliptin. Myopathy may occasionally present as rhabdomyolysis, with or without acute renal failure, secondary to myoglobinuria; fatalities have occurred rarely.
Physicians should use caution when prescribing Gliptar® to patients with predisposing factors for rhabdomyolysis. Before starting treatment, patients should have their creatine kinase levels measured:
with impaired kidney function;
with uncontrolled hypothyroidism;
with hereditary muscle diseases in personal or family history;
with a history of muscle toxicity from statins or fibrates;
with alcohol addiction;
Elderly (≥65 years): the need for CK measurement should be considered in the presence of other predisposing factors for rhabdomyolysis;
female.
The risks of treatment with the drug should be considered in relation to the potential benefits.
Others
The medicinal product Gliptar®, tablets, contains lactose. Gliptar® is contraindicated in patients with rare hereditary conditions: lactose intolerance, lactase deficiency or glucose-galactose malabsorption.
This medicinal product contains less than 1 mmol (less than 23 mg) sodium/dose, i.e. essentially sodium-free.
Use during pregnancy or breastfeeding
Pregnancy
There are no adequate studies of the use of vildagliptin in pregnant women.
Animal studies have shown reproductive toxicity at high doses. The potential risk to humans is unknown. In the absence of data, vildagliptin should not be used during pregnancy.
Breastfeeding period
It is not known whether vildagliptin is excreted in human milk. Animal studies have shown the presence of vildagliptin in animal milk. Gliptar® should not be administered to women who are breastfeeding.
Fertility
No studies have been conducted on the effect of the drug Gliptar® on human fertility.
Ability to influence reaction speed when driving vehicles or other mechanisms
Studies on the effect of the drug on the ability to drive and use machines have not been conducted. Patients who experience dizziness should not drive or use machines.
Method of administration and doses
The dose of the drug Gliptar® should be selected individually.
Monotherapy
The recommended dose of Gliptar® when used as monotherapy is 50 mg once or twice daily.
Combination therapy
In combination with insulin (with or without metformin), the recommended dose of Gliptar® is 50 mg once or twice daily depending on renal function (see section “Dosage for patients with hepatic or renal impairment” below).
In combination with metformin or in combination with metformin and a sulfonylurea, the recommended dose of Gliptar® is 50 mg twice daily.
In combination with a sulfonylurea or thiazolidinedione, the recommended dose of Gliptar® is 50 mg once daily.
When combined with a sulfonylurea, a lower dose of the sulfonylurea may be considered to reduce the risk of hypoglycemia.
Dosages greater than 50 mg twice daily are not recommended.
The tablets can be taken regardless of meals.
Dosage for patients with impaired liver or kidney function
Gliptar® is not recommended for use in patients with impaired liver function, including patients with pre-treatment ALT or AST levels greater than 2.5 times the upper limit of normal.
For patients with mild renal impairment (creatinine clearance ≥ 50 ml/min, corresponding to serum creatinine ≤ 150 μmol/l in men and ≤ 133 μmol/l in women), no dose adjustment of Gliptar® is required. For patients with moderate or severe renal impairment or end-stage renal disease (ESRD), the recommended dose is 50 mg once daily.
Dosage for elderly patients
No dose adjustment is required for elderly patients.
Children
The use of the drug Gliptar® is not recommended for children and adolescents under 18 years of age due to the lack of data on safety and efficacy.
Overdose
Information on overdose of the drug Gliptar® is limited.
Data on possible symptoms of overdose were obtained during a dose escalation study in healthy volunteers who took vildagliptin for 10 days. At a dose of 400 mg, three cases of muscle pain were observed, as well as several cases of mild and transient paresthesia, fever, edema, and transient elevations in lipase levels. At a dose of 600 mg, one volunteer developed edema of the legs and arms, a significant increase in creatinine phosphokinase (CPK) levels, accompanied by increases in AST, C-reactive protein, and myoglobin. Three volunteers in this group had edema of both legs, accompanied in two cases by paresthesia. All symptoms and laboratory abnormalities resolved after discontinuation of the study drug.
Treatment
In case of overdose, supportive therapy is recommended. Vildagliptin is not removed by hemodialysis, however, most of the hydrolysis metabolites (LAY 151) can be removed by hemodialysis.
Side effects
Safety data were obtained in controlled studies of at least 12 weeks duration in a total of 3784 patients who received vildagliptin at a daily dose of 50 mg (once a day) or 100 mg (50 mg twice a day or 100 mg once a day). Of these patients, 2264 patients received vildagliptin as monotherapy and 1520 patients received vildagliptin in combination with another medicinal product. 2682 patients were treated with vildagliptin at a dose of 100 mg per day (either 50 mg twice a day or 100 mg once a day), and 1102 patients were treated with vildagliptin at a dose of 50 mg once a day.
Most adverse reactions that occurred with vildagliptin were mild in nature and transient and did not require discontinuation of treatment. No relationship was found between the development of adverse reactions and the age or race of the patient, the duration of drug administration, or the daily dose.
Isolated cases of hepatic dysfunction (including hepatitis) have been reported. These cases were usually asymptomatic, without clinical sequelae, and liver function tests (LFTs) returned to normal after discontinuation of treatment. In controlled monotherapy and add-on therapy studies of up to 24 weeks duration, the incidence of elevations of ALT or AST ≥ 3 times the upper limit of normal (detected on two consecutive measurements or at the final visit) was 0.2%, 0.3% and 0.2% for vildagliptin 50 mg once daily, twice daily and all comparators, respectively. Transaminase elevations were mostly asymptomatic, did not progress and were not associated with cholestasis or jaundice.
Isolated cases of angioedema reported with vildagliptin were observed at a similar frequency to that seen in the control group. A higher rate was observed in the group where vildagliptin was used in combination with an angiotensin-converting enzyme (ACE) inhibitor. Most events were mild in severity and resolved with vildagliptin therapy.
Adverse reactions observed during double-blind studies in patients taking vildagliptin as monotherapy and in combination therapy are listed below for each indication by system organ class and absolute frequency. The frequency is defined as follows: very common (≥ 1/10), common (≥ 1/100, < 1/10), uncommon (≥ 1/1,000, < 1/100), rare (> 10,000, ≤ 1/1,000), very rare (≤ 1/10,000), unknown frequency (cannot be estimated from the available data). Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Vildagliptin Monotherapy: Adverse reactions reported in patients receiving vildagliptin 100 mg/day as monotherapy in double-blind studies (N = 1855).
Infections and infestations: very rare - upper respiratory tract infection, nasopharyngitis.
Metabolic: infrequently - hypoglycemia.
From the nervous system: often - dizziness, tremor; infrequently - headache, fatigue.
Cardiovascular system: infrequently - peripheral edema.
Gastrointestinal: infrequently - constipation.
Musculoskeletal and connective tissue disorders: infrequently - arthralgia.
Description of selected adverse reactions.
In controlled studies of vildagliptin monotherapy, the overall incidence of early discontinuation of treatment due to adverse reactions was not higher in patients treated with vildagliptin 100 mg/day (0.3%) than in patients treated with placebo (0.6%) or comparators (0.5%).
In comparative controlled monotherapy studies, uncommon cases of hypoglycaemia were observed in 0.4% (7 out of 1855) of patients treated with vildagliptin 100 mg daily compared to 0.2% (2 out of 1082) of patients treated with active comparator or placebo, with no serious or severe events.
Clinical studies of up to 2 years duration did not reveal any additional signs of danger or unforeseen risks with vildagliptin monotherapy.
Combination with metformin: Adverse reactions reported in patients receiving vildagliptin 100 mg/day in combination with metformin in double-blind studies (N = 208).
Metabolic: often - hypoglycemia.
From the nervous system: often - tremor, dizziness, headache; infrequently - fatigue.
Gastrointestinal: often - nausea.
Description of selected adverse reactions.
In controlled clinical trials of the combination of vildagliptin 100 mg/day with metformin, no discontinuation of treatment due to adverse reactions was reported in either the vildagliptin 100 mg/day + metformin group or the placebo + metformin group.
In clinical trials, hypoglycemia was observed commonly in patients receiving vildagliptin 100 mg/day in combination with metformin (1%) and uncommonly in patients receiving placebo + metformin (0.4%). No cases of severe hypoglycemia were reported in the vildagliptin groups.
In clinical trials, when vildagliptin 100 mg/day was added to metformin, the body weight of patients did not change compared to baseline (+0.2 kg and –1.0 kg in the vildagliptin and placebo groups, respectively).
In clinical trials of up to 2 years or more, no additional safety or unexpected risks were identified when vildagliptin was added to metformin.
Combination with a sulfonylurea: Adverse reactions reported in patients receiving vildagliptin 50 mg in combination with a sulfonylurea in double-blind studies (N = 170).
Infections and infestations: very rarely - nasopharyngitis.
Metabolic: often - hypoglycemia.
From the nervous system: often - tremor, dizziness, headache, asthenia.
Gastrointestinal: infrequently - constipation.
Description of selected adverse reactions.
In controlled clinical trials of the combination of vildagliptin 50 mg and a sulfonylurea, the overall incidence of early discontinuation of treatment due to adverse reactions was 0.6% in the combination of vildagliptin 50 mg with a sulfonylurea versus 0% in the placebo + sulfonylurea treatment group.
In clinical trials, the incidence of hypoglycemia when vildagliptin 50 mg once daily was added to glimepiride was 1.2% versus 0.6% in the placebo + glimepiride group. No severe hypoglycemia was reported in the vildagliptin treatment groups.
In clinical studies, when vildagliptin 50 mg/day was added to glimepiride, the body weight of patients did not change compared to baseline (–0.1 kg and –0.4 kg in the vildagliptin and placebo groups, respectively).
Combination with a thiazolidinedione. Adverse reactions reported in patients receiving vildagliptin 100 mg daily in combination with a thiazolidinedione in double-blind studies (N = 158).
Metabolic: often - weight gain; infrequently - hypoglycemia.
From the nervous system: infrequently - headache, asthenia.
Cardiovascular system: often - peripheral edema.
Description of selected adverse reactions.
In controlled clinical trials of the combination of vildagliptin 100 mg/day and a thiazolidinedione, no early discontinuation of treatment due to adverse reactions was reported in either the vildagliptin 100 mg/day and thiazolidinedione treatment group or the placebo + thiazolidinedione treatment group.
In clinical trials, hypoglycemia was uncommon in patients treated with vildagliptin + pioglitazone (0.6%) but common in patients treated with placebo + pioglitazone (1.9%). No severe cases of hypoglycemia were reported in the vildagliptin treatment groups.
In the pioglitazone add-on therapy study, the absolute weight gain with placebo and vildagliptin 100 mg daily was 1.4 and 2.7 kg, respectively.
The percentage of peripheral edema when vildagliptin 100 mg daily was added to the maximum dose of pioglitazone used as background medication (45 mg once daily) was 7.0% compared to 2.5% when pioglitazone was used alone as background medication.
Combination with metformin and a sulfonylurea: Adverse reactions reported in patients receiving vildagliptin 50 mg twice daily in combination with metformin and a sulfonylurea (N = 157).
Metabolic: often - hypoglycemia.
From the nervous system: often - dizziness, tremor.
Skin and subcutaneous tissue disorders: often - hyperhidrosis.
General disorders: often - asthenia.
Description of selected adverse reactions.
Hypoglycemia was common in both treatment groups (5.1% in the vildagliptin + metformin + glimepiride group compared to 1.9% in the placebo + metformin + glimepiride group). One severe hypoglycemic event was reported in the vildagliptin group.
At the end of the study, the effect on mean body weight was neutral (+0.6 kg in the vildagliptin group and -0.1 kg in the placebo group).
Combination with insulin: Adverse reactions reported in patients receiving vildagliptin 100 mg/day in combination with insulin (with or without metformin) in double-blind studies (N = 371).
Metabolic: often - decreased blood glucose level.
From the nervous system: often - headache, chills.
On the part of the gastrointestinal tract: often - nausea, gastroesophageal reflux disease; infrequently - diarrhea, flatulence.
Description of selected adverse reactions.
In controlled clinical trials with vildagliptin 50 mg twice daily in combination with insulin, with or without concomitant metformin, the overall incidence of early discontinuation of treatment due to adverse reactions was 0.3% in the vildagliptin group, while no early discontinuation due to adverse reactions was observed in the placebo group.
The incidence of hypoglycemia was similar in both treatment groups (14.0% in the vildagliptin group compared to 16.4% in the placebo group). Severe hypoglycemia was observed in 2 patients in the vildagliptin group and 6 patients in the placebo group.
At the end of the study, the effect on mean body weight was neutral (body weight changed by +0.6 kg from baseline in the vildagliptin treatment group and remained unchanged in the placebo group).
Post-marketing experience.
Gastrointestinal tract: frequency unknown - pancreatitis.
On the part of the hepatobiliary system: frequency unknown - hepatitis (reversible after discontinuation of the drug), deviations in liver function tests from the norm (reversible after discontinuation of the drug).
Musculoskeletal and connective tissue disorders: frequency unknown - myalgia, rhabdomyolysis.
Skin and subcutaneous tissue disorders: frequency unknown - urticaria, bullous and exfoliative skin lesions, including bullous pemphigoid.
Reporting adverse reactions after registration of a medicinal product is important. This allows monitoring of the benefit/risk ratio of the medicinal product. Medical and pharmaceutical professionals, as well as patients or their legal representatives, should report all cases of suspected adverse reactions and lack of efficacy of the medicinal product via the automated pharmacovigilance information system at the following link: https://aisf.dec.gov.ua
Expiration date
3 years.
Storage conditions
In the original packaging, at a temperature not exceeding 25 ° C. Keep out of the reach of children.
Packaging
10 tablets in a blister, 6 blisters in a pack.
Vacation category
According to the recipe.
Producer
PJSC "Kyivmedpreparat"
Location of the manufacturer and its business address.
Ukraine, 01032, Kyiv, Saksaganskoho St., 139.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.