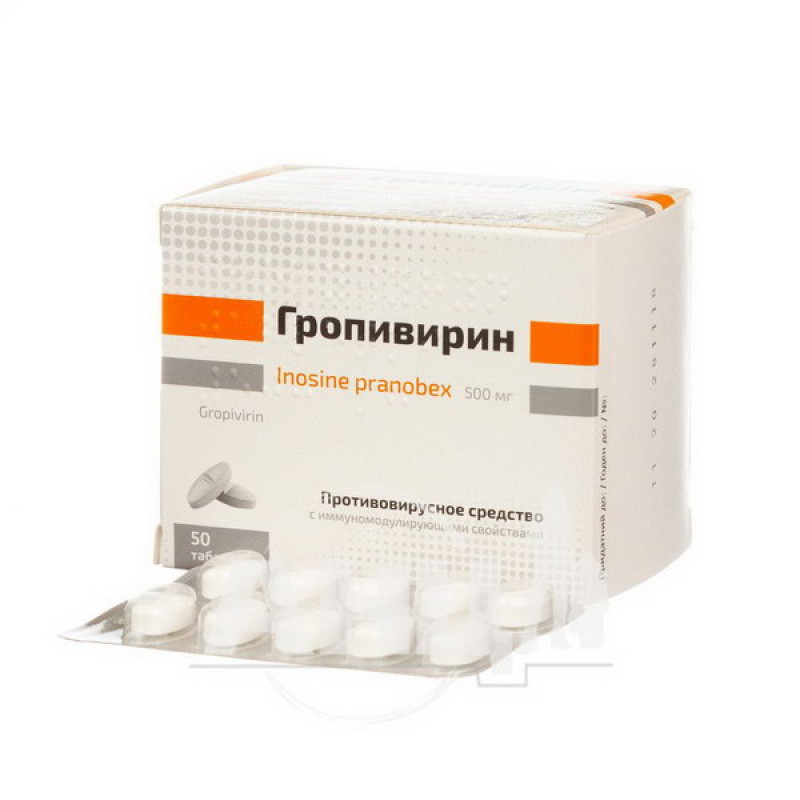
Gropivirine tablets 500 mg blister No. 50

Instructions for use Gropivirin tablets 500 mg blister No. 50
Composition
active ingredient: inosine pranobex;
1 tablet contains 500 mg of inosine pranobex;
excipients: corn starch, mannitol (E 421), povidone (Kollidon 25), magnesium stearate.
Dosage form
Pills.
Main physicochemical properties: tablets from almost white to yellowish-white in color, oval, biconvex, oblong in shape, with a score on one side, with a slight specific odor.
Pharmacotherapeutic group
Antiviral drugs for systemic use.
ATX code J05A X05.
Pharmacological properties
Pharmacodynamics
Gropivirine is an antiviral agent with immunomodulatory properties. The drug normalizes (to an individual norm) the deficiency or dysfunction of cellular immunity, inducing the maturation and differentiation of T-lymphocytes and T1-helpers, potentiating the induction of a lymphoproliferative response in mitogenic or antigen-active cells. Gropivirine models the cytotoxicity of T-lymphocytes and natural killers, the function of T8-suppressors and T4-helpers, and also increases the amount of immunoglobulin G and surface markers of complement. Gropivirine increases the synthesis of interleukin-1 (IL-1) and the synthesis of interleukin-2 (IL-2), regulates the expression of IL-2 receptors. Gropivirine significantly increases the secretion of endogenous gamma-interferon and reduces the production of interleukin-4 in the body. Gropivirine enhances the action of neutrophil granulocytes, chemotaxis and phagocytosis of monocytes and macrophages. Gropivirine inhibits virus synthesis by incorporating inosine-orotic acid into the polyribosomes of virus-infected cells and inhibits the addition of adenylic acid to viral mRNA.
Pharmacokinetics
After taking the drug orally at a dose of 1.5 g, the maximum concentration of inosine pranobex in the blood plasma is reached after 1 hour and is 600 μg/ml. In the body, inosine pranobex is metabolized in the liver with the formation of uric acid. The half-life of 4-(acetylamino)benzoate is 50 min, 1-(dimethylamino)-2-propanol - 3.5 hours. It is excreted by the kidneys in the form of metabolites.
Indication
viral infections caused by herpes simplex virus types 1 and 2, varicella virus, cytomegalovirus, Epstein-Barr virus, measles virus, mumps virus, including in patients with immunodeficiency states; viral respiratory infections; papillomavirus infections of the skin and mucous membranes: genital warts, papillomavirus infection of the vulva, vagina and cervix (as part of complex therapy); acute viral encephalitis (as part of complex therapy); viral hepatitis (as part of complex therapy); subacute sclerosing panencephalitis (as part of complex therapy).
Contraindication
Hypersensitivity to any component of the drug, gout, hyperuricemia.
Interaction with other medicinal products and other types of interactions
The drug should not be taken simultaneously with immunosuppressants. Caution should be exercised when prescribing the drug with xanthine oxidase inhibitors and agents that promote the excretion of uric acid, including diuretics - thiazide diuretics (such as hydrochlorothiazide, chlorthalidone, indapamide) and loop diuretics (such as furosemide, torasemide, ethacrynic acid).
When used simultaneously with azidothymidine, nucleotide formation is increased due to increased bioavailability of azidothymidine in blood plasma and increased intracellular phosphorylation in human blood monocytes.
Application features
Since during treatment with Gropivirine, a temporary increase in serum uric acid levels is possible, especially in men and the elderly, the drug should not be used in patients suffering from gout, hyperuricemia, and should be used with caution in patients with urolithiasis and reduced kidney function. When using the drug for more than 3 months, it is advisable to check laboratory indicators of liver and kidney function (transaminases, creatinine), serum uric acid levels, and perform a blood test every month.
Some individuals may experience acute hypersensitivity reactions (angioedema, anaphylactic shock, urticaria). In such cases, Gropivirine therapy should be discontinued.
With prolonged use of the drug, there is a risk of developing nephrolithiasis.
Ability to influence reaction speed when driving vehicles or other mechanisms
The effect of the drug on the reaction speed when driving or operating other mechanisms has not been studied. However, patients should be aware that the drug may cause dizziness or other adverse reactions from the nervous system.
Use during pregnancy or breastfeeding
Studies on the possibility of fetal malformations and impaired fertility in humans have not been conducted. It is not known whether inosine pranobex passes into breast milk. It is not recommended to use the drug during pregnancy and breastfeeding.
Method of administration and doses
The drug is administered orally.
Adults and children over 12 years of age: 50 mg/kg body weight (usually 6-8 tablets divided into 3-4 doses), maximum daily dose – 4 g.
Children aged 1 to 12 years: 50 mg/kg body weight (usually 1 tablet per 10 kg body weight for a child weighing 10–20 kg, with a body weight of more than 20 kg, prescribe the dose as for adults) in 3–4 doses per day, maximum daily dose – 4 g. To facilitate swallowing, the tablet can be crushed.
Duration of treatment.
Acute diseases: for diseases with a short course, the course of treatment is from 5 to 14 days. After the symptoms of the disease have subsided, treatment should be continued for another 1-2 days or longer, depending on the course of the disease and the patient's condition.
Viral diseases with a long course: treatment should be continued for 1-2 weeks after the symptoms of the disease have subsided or longer, depending on the course of the disease and the patient's condition.
Recurrent diseases: at the initial stage of treatment, the same recommendations as for acute diseases apply. During maintenance therapy, the dose can be reduced to 500-1000 mg (1-2 tablets) per day. At the first signs of relapse, it is necessary to resume taking the daily dose recommended for acute diseases, and this dose should be continued for 1-2 days after the symptoms disappear. The course of treatment can be repeated several times on the recommendation of a doctor, depending on the patient's condition.
Chronic diseases: the drug is used in a daily dose of 50 mg/kg of body weight according to the following schemes:
asymptomatic diseases – take for 30 days with a break of 60 days;
diseases with moderately severe symptoms – take for 60 days with a break of 30 days;
Diseases with severe symptoms – use for 90 days with a 30-day break.
The course of treatment should be repeated as many times as necessary, with constant monitoring of the patient's condition and indications for extending therapy.
For infections caused by human papillomavirus (external genital warts (condyloma acuminatum) or papillomavirus infection of the cervical canal), take 3 g (2 tablets 3 times a day) for 14-28 days as monotherapy or as an adjunct to local therapy or surgical treatment according to the following regimens:
for the treatment of patients from the low-risk group (patients with normal immunity or patients with a low risk of relapse), the drug is used for 14-28 days until maximum eradication of the virus is achieved, then a break of 2 months should be taken. The course of treatment can be repeated using the same dose, while it is necessary to constantly monitor the patient's condition and indications for prolonging therapy; for the treatment of patients from the high-risk group* (patients with immunodeficiency or with a high risk of relapse), the drug is used 5 days a week, consecutively 1-2 weeks a month for 3 months. The course of treatment should be repeated as many times as necessary, while it is necessary to constantly monitor the patient's condition and indications for prolonging therapy.
* High-risk factors in patients with recurrent or recurrent cervical dysplasia or genital HPV infection, as with other similar diseases, include:
Immunodeficiency caused by: a history of chronic or recurrent infections or sexually transmitted diseases; chemotherapy; chronic alcoholism; long-term use of oral contraceptives (2 years or longer); folate level in erythrocytes less than 660 nmol/l; multiple sexual partners or change of permanent sexual partner; frequent vaginal intercourse (≥ 2–6 times per week) or anal sex; atopy (hereditary predisposition to increased sensitivity); poorly controlled diabetes; smoking; genital papillomavirus infection lasting more than 2 years or with 3 or more recurrences in history; the patient had skin warts in childhood.
In subacute sclerosing panencephalitis, the daily dose is 100 mg/kg of body weight, the maximum dose is 3–4 g/day, while it is necessary to constantly monitor the patient's condition and indications for prolonging therapy.
Children
The drug is used in children aged 1 year and older.
Overdose
No cases of overdose have been observed. Overdose may cause an increase in uric acid levels in the blood serum and urine. Treatment is symptomatic.
Adverse reactions
During treatment with Gropivirine, the only persistent side effect of the drug in adults and children is a temporary increase in uric acid levels in the blood serum and urine, which return to their original normal values a few days after the end of treatment.
Laboratory tests: increased blood uric acid levels, increased urine uric acid levels, increased blood urea nitrogen levels, increased transaminase levels, increased blood alkaline phosphatase levels.
General disorders: fatigue, malaise.
Skin and subcutaneous tissue disorders: rash, itching.
On the part of the gastrointestinal tract: vomiting, nausea, feeling of discomfort in the epigastric region of the abdomen, diarrhea, constipation.
From the nervous system: headache, dizziness, drowsiness, sleep disorders.
Musculoskeletal and musculoskeletal disorders: arthralgia.
Mental disorders: nervousness.
Other adverse reactions have also been reported:
from the digestive tract: abdominal pain (in the upper part);
from the immune system: anaphylactic reactions, anaphylactic shock, angioedema, hypersensitivity, urticaria;
from the nervous system: dizziness;
Skin and subcutaneous tissue disorders: erythema.
Expiration date
2 years.
Do not use the drug after the expiration date indicated on the package.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 °C.
Keep out of reach of children.
Packaging
10 tablets in a blister, 5 blisters in a pack.
Vacation category
According to the recipe.
Producer
JSC "Farmak".
Location of the manufacturer and its business address
Ukraine, 04080, Kyiv, Kyrylivska St., 74.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.