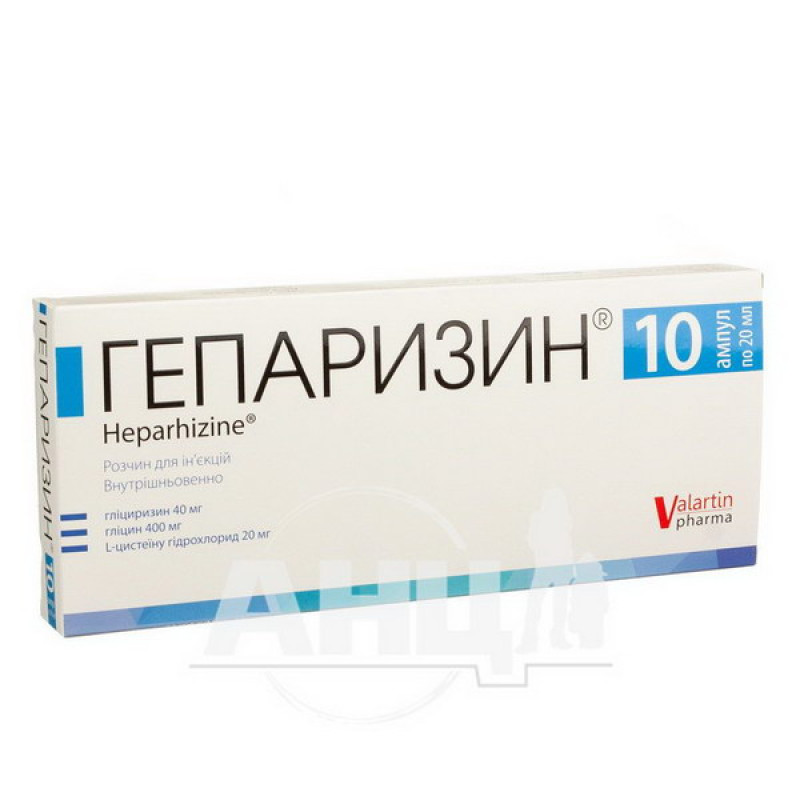
Heparizin solution for injection ampoule 20 ml No. 10
Instructions Heparizin solution for injection ampoule 20 ml No. 10
Composition
active ingredients:
1 ampoule contains: monoammonium glycyrrhizinate equivalent to glycyrrhizin 40 mg, glycine 400 mg, L-cysteine hydrochloride 20 mg;
Excipients: anhydrous sodium sulfite, sodium chloride, water for injection, concentrated ammonia solution.
Dosage form
Solution for injection.
Main physicochemical properties: colorless transparent solution.
Pharmacotherapeutic group
Drugs used in liver diseases, lipotropic substances. Hepatotropic drugs. ATX code A05B A.
Pharmacological properties
Pharmacodynamics.
Anti-inflammatory effect
Antiallergic: Glycyrrhizin can inhibit the development of allergic reactions such as Arthus phenomenon and Schwarzman phenomenon in animals. This drug can cause stress-induced suppression of cortical hormone and at the same time inhibit the granulation and atrophy of thymus under the action of this hormone. This drug does not affect the exudation effect of the hormone.
Blockade of metabolic enzymes of the arachidonic acid cycle. Glycyrrhizin can bind to phospholipase A2 (which is an activator of the arachidonic acid cycle) and lipoxygenase (which affects arachidonic acid and the synthesis of inflammatory mediators induced by it), due to which glycyrrhizin selectively inhibits the phosphorylation of these enzymes, and therefore inhibits their activation.
Immunoregulatory effects
In in vitro studies, glycyrrhizin:
regulates T-cell activation;
induces interferon-γ;
activates NK cells;
promotes differentiation of extra-thymic T lymphocytes.
Inhibitory effect on experimental hepatocyte damage
In vitro, glycyrrhizin is able to prevent damage to primary cultures of animal hepatocytes.
Proliferative effect on hepatocytes
In vitro studies using primary hepatocyte cultures have shown that glycyrrhizic and glycyrrhetinic acids stimulate the proliferation of primary hepatocyte cultures.
Inhibition of virus spread and virus deactivation.
In experimental conditions on animals infected with mouse hepatitis virus (MHV), administration of glycyrrhizin helps prolong the life of the animals.
In animals infected with the cowpox virus, glycyrrhizin may prevent the development of smallpox.
Other in vitro experiments further demonstrated an inactivating effect on the virus, as well as an inhibitory effect on the spread of the virus.
Glycine and cysteine hydrochloride can suppress or reduce the manifestations of pseudoaldosteronism caused by metabolic disorders of electrolyte metabolism with prolonged use of glycyrrhizin.
Pharmacokinetics.
Blood concentration
In studies involving healthy volunteers, after intravenous administration of 40 ml of the drug (corresponding to 80 mg of glycyrrhizin), the concentration in the blood after an initial increase in the next 10 hours decreases rapidly. Then it decreases gradually. Glycyrrhizic acid (a hydrolyzed metabolite of glycyrrhizin) is detected in the blood 6 hours after administration, reaching a maximum 24 hours after administration, and is almost completely excreted after 48 hours.
Urinary excretion
In studies with healthy volunteers, after intravenous administration, the concentration of glycyrrhizin in the urine decreases gradually. The excretion volume is on average 1.2% of the dose taken 27 hours after administration. Glycyrrhizic acid is detected 6 hours after administration and reaches peak concentration after 22-27 hours.
Pharmacokinetics in animals
Distribution. Analysis of internal organs was started 10 minutes after intravenous administration of 3H-glycyrrhizin to animals. Glycyrrhizin is distributed throughout all internal organs of animals. The liver accumulates the largest amount of the administered drug (73%). Drug accumulation decreases in the following organs (listed in descending order): kidneys, lungs, heart, adrenal glands.
Indication
Improving impaired liver function in chronic liver diseases.
Contraindication
Hypersensitivity to the components of the drug.
The drug is contraindicated in patients with aldosteronism, myopathy or hypokalemia (there is a possibility of exacerbation of hypokalemia, hypertension).
Interaction with other medicinal products and other types of interactions
Loop diuretics (ethacrynic acid, furosemide), thiazide diuretics and other antihypertensive diuretics (trichlormethiazide, trichlorothalidone) enhance the effect of glycyrrhizin on the excretion of potassium, thereby reducing the concentration of potassium in the blood serum. Given the likelihood of hypokalemia (atonic sensations, muscle weakness), appropriate monitoring is necessary, including determination of the concentration of potassium in the blood serum.
Against the background of a decrease in serum potassium concentration as a result of potassium excretion under the influence of glycyrrhizic acid contained in this drug, the action of moxifloxacin hydrochloride may cause the development of ventricular tachycardia (including torsades de pointes) and long QT syndrome.
Application features
Use with caution.
The possibility of rhabdomyolysis has been reported with oral administration of glycyrrhizin or other drugs containing glycyrrhizin.
General precautions
A detailed history should be taken before use to prevent the development of hypersensitivity reactions.
The necessary equipment should be prepared for first aid in the event of hypersensitivity reactions.
The patient should remain at rest after administration of the drug. The patient requires close observation after administration of the drug.
The development of pseudoaldosteronism is possible with simultaneous use with other drugs containing glycyrrhizin.
During intravenous injection, it is necessary to monitor the patient's condition and reduce the rate of drug administration to a minimum. The integrity of the ampoule can be violated only after its disinfection with an alcohol solution.
Use in elderly patients
The drug should be administered with caution to elderly patients, as there is a high probability of developing hypokalemia.
This medicinal product contains 45 mg/dose of sodium. Caution should be exercised when used in patients on a controlled sodium diet.
Due to the content of sodium sulfite, the drug may rarely cause hypersensitivity reactions and bronchospasm.
Use during pregnancy or breastfeeding
Not used because safety and efficacy have not been established.
Ability to influence reaction speed when driving vehicles or other mechanisms
Does not affect the ability to drive vehicles or other mechanisms.
Method of administration and doses
40-60 ml of the drug should be administered by intravenous injection or infusion once a day. The doctor may adjust the dose depending on the patient's age and symptoms of the disease. The maximum daily dose should not exceed 100 ml.
Children.
Studies on the effectiveness and safety of the drug for children have not been conducted.
Overdose
Data is missing.
Side effects
Adverse reactions, the development of which was observed most often
Shock and anaphylactic shock (frequency unknown): shock or anaphylactic shock may develop (including a sudden decrease in blood pressure, loss of consciousness, shortness of breath, cardiopulmonary failure, flushing, facial edema). In view of the above, patients require careful monitoring. If symptoms of an adverse reaction appear, the drug should be discontinued immediately and appropriate treatment should be prescribed.
Anaphylactic reactions (frequency unknown): Anaphylaxis (including shortness of breath, flushing and facial swelling) may occur. In view of the above, patients require close monitoring. If symptoms of an adverse reaction occur, the drug should be discontinued immediately and appropriate treatment should be prescribed.
Pseudoaldosteronism (frequency unknown): Increasing the dose or long-term and continuous administration of the drug may lead to symptoms of pseudoaldosteronism, such as severe hypokalemia, increased frequency of hypokalemia, increased blood pressure, sodium and fluid retention, edema, weight gain. Careful monitoring (in particular, determination of serum potassium concentration) is necessary. If symptoms of an adverse reaction appear, the drug should be discontinued immediately.
In addition, hypokalemia can cause the development of general weakness or moderate muscle weakness.
Other adverse reactions
The following adverse reactions may occur with increasing dose and may lead to a decrease in serum potassium and an increase in blood pressure.
Frequency Adverse reaction | 0.1-5% | Less than 0.1% | Frequency unknown |
| On the part of the immune system | - | Rash | Hives, itching |
| Electrolyte exchange | Hypokalemia, increased blood pressure | Swelling, fatigue, pain in the muscles | - |
| From the digestive system | - | Feeling of discomfort in the epigastric region | Nausea, vomiting |
| Respiratory system | - | - | Cough |
| From the organs of vision | - | - | Transient visual disturbance (blurred vision, photopsia) |
| Others | - | Eczema, skin discomfort, headache, fever, myalgia, malaise, paresthesia (numbness, tingling sensation), fever, hyperventilation syndrome (feeling of heat in the shoulders, feeling of cold in the extremities, cold sweat, dry mouth, palpitations), glycosuria | Hot flashes, dysthymia |
Expiration date
3 years.
Storage conditions
Store in original packaging at a temperature not exceeding 25 ° C. Keep out of the reach of children.
Incompatibility
The drug is prohibited from mixing with other medications.
Packaging
20 ml of solution for injection in an ampoule, 10 ampoules in a cardboard box.
Vacation category
According to the recipe.
Producer
Beijing Kawin Technology Share-Holding Co., Ltd.
Ukraine, 08135, Kyiv region, Kyiv-Svyatoshynskyi district, Chaykyi village, Hrushevskoho str., 60.
Applicant
LLC "VALARTIN PHARMA".
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.