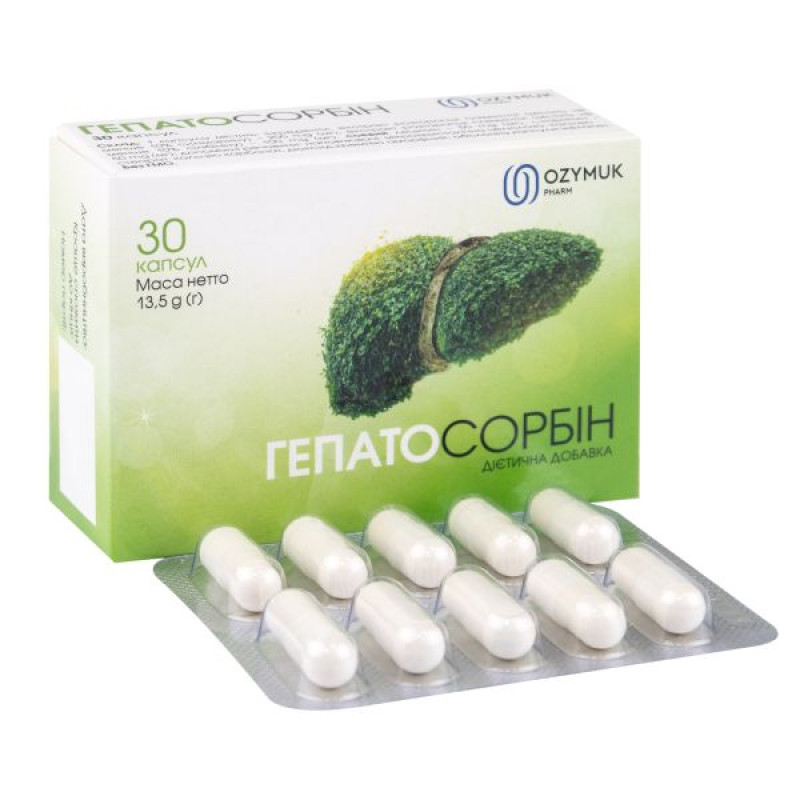
Hepatosorbin capsules No. 30

composition
1 capsule contains ingredients: milk thistle extract (contains not less than 10% silymarin) - 200 mg (mg), milk thistle extract (contains not less than 10% silibinin) - 100 mg (mg), soy lecithin - 50 mg (mg) L-Carnitine - 50 mg (mg); excipients: microcrystalline cellulose, calcium stearate, calcium carbonate, amorphous silicon dioxide; capsule shell: gelatin.
Does not contain GMOs.
Indications for use
Hepatosorbin can be recommended as a dietary supplement to the diet. It is a source of milk thistle extract, soy lecithin and L-carnitine, which are involved in the normalization of the functional state and restoration of physiological functions of the liver, disrupted due to the negative effects of toxic substances, alcohol, viruses and other factors.
It is recommended to consult a doctor before use.
Dosage and administration.
Take 1 capsule 2 times a day, regardless of meals.
Duration of use: 3-4 months. Further duration of use should be agreed with the doctor.
It is not a medicine.
WARNING:
Do not exceed the recommended daily intake and do not use as a dietary supplement. Do not use the dietary supplement in case of individual intolerance to the components, during pregnancy and breastfeeding; intended for adults only.
Date of manufacture:
indicated on the packaging (if applicable).
Expiration date
24 months from the date of manufacture.
"Best before end use": stated on the packaging.
Batch number: indicated on the packaging.
Release form
1 capsule with a net weight of 450 mg (mg) ±7%; capsules. 30 (with a net weight of 13.5 g (g) ± 7%, 10 capsules in a blister, 3 blisters in a cardboard box).
Storage conditions
Store in the original packaging in a dry place, protected from light and out of reach of children at a temperature of +4°C to +25°C.
Manufacturer's name, address and telephone number: Indicated on the packaging.
Name and location of the food market operator responsible for food information: indicated on the packaging.
Name and location of the company responsible for approving consumer claims: indicated on the packaging.
Barcode: indicated on the packaging.
Mark of goods and services (if any): indicated on the packaging.
TU 10.8-41979246-002: 2020
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.