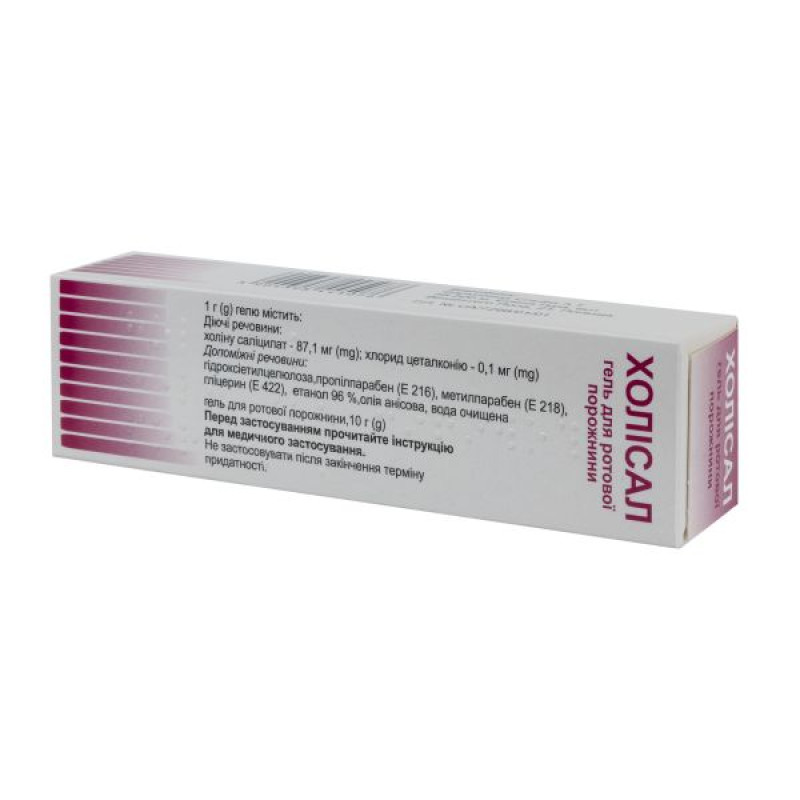
Holisal oral gel tube 10 g
Instructions for use: Holisal oral gel, tube 10 g
Composition
active ingredients: choline salicylate; cetalkonium chloride;
1 g of gel contains: choline salicylate 87.1 mg and cetalkonium chloride 0.1 mg;
excipients: hydroxyethyl cellulose, methylparaben (E 218), propylparaben (E 216), glycerin (E 422), anise oil, ethanol 96%, purified water.
Dosage form
Oral gel.
Main physicochemical properties: transparent, colorless, homogeneous mass with the smell of anise oil.
Pharmacotherapeutic group
Means for topical use in dentistry.
ATX code A01A D.
Pharmacological properties
Pharmacodynamics
The drug has anti-inflammatory, analgesic and antiseptic effects. The main active ingredient in the drug - choline salicylate - has a local anti-inflammatory and weak analgesic effect. The mechanism of analgesic, antipyretic and anti-inflammatory action of salicylates is associated with the inhibition of cyclooxygenase activity, as well as the activity of thromboxane synthetase. As a result of this action, the synthesis of prostanoids is inhibited, primarily prostaglandins E and P in tissues changed by inflammation, in the wall of the digestive tract and in the kidneys. When applied topically, in usual doses, it does not have a general effect. Cetalkonium chloride enhances the analgesic and anti-inflammatory effect of the drug. Antiseptics that are part of the gel base, methylparaben and propylparaben (in concentrations of 0.15% and 0.08%, respectively), have an antibacterial effect.
Pharmacokinetics
Choline salicylate is rapidly absorbed from mucous membranes (approximately 2 times faster than acetylsalicylic acid). Salicylates easily penetrate most tissues and body fluids. They have a high affinity for blood proteins and tissues, binding in the range of 50% to 80% with blood plasma albumin. The half-life of salicylates is from 2 to 4 hours. Biotransformation of salicylates occurs in tissues, mainly they are transformed in the liver to salicylic acid, from which salicylouric acid, glucuronides, acetal, and also gentisic acid are formed. The resulting biotransformation products are excreted in the urine (75% is salicylouric acid). Approximately 10% of salicylates are excreted unchanged in the urine. Excretion occurs slowly, up to 24 hours approximately 50% of the dose is excreted.
Indication
Inflammatory processes of the oral mucosa; erosions and ulcers of the oral cavity; gingivitis; periodontitis.
Contraindication
Hypersensitivity to choline salicylate, other salicylates, cetalkonium chloride or to any other components of the drug.
Interaction with other medicinal products and other types of interactions
When applied topically at recommended dosages, there is no risk of interaction.
Only in the case of a significant overdose and the development of symptoms of systemic effects of choline salicylate should it be taken into account that it acts synergistically with other anti-inflammatory, antipyretic and analgesic drugs.
Application features
The product contains methylparaben and propylparaben, which may cause irritation. If any adverse reactions occur, discontinue use and consult a doctor.
Ability to influence reaction speed when driving vehicles or other mechanisms
The drug does not impair psychophysical performance and does not affect the ability to drive vehicles or work with other mechanisms.
Use during pregnancy or breastfeeding
No studies have been conducted on the duration and extent of absorption of choline salicylate in the body after topical application of the gel to the mucous membranes of the oral cavity. In the I-II trimesters of pregnancy, the drug should be used with caution, taking into account the risk/benefit ratio. Salicylic acid derivatives are contraindicated in the III trimester of pregnancy. The active substances penetrate into breast milk, therefore it is not recommended to use the drug during breastfeeding.
Method of administration and doses
Apply a small amount of gel to the affected area and rub gently for 2 minutes. Apply 2-3 times a day. Squeeze a strip of gel 1 cm long for adults and 0.5 cm for children onto a clean finger and gently rub into the affected area of the mucous membrane for several minutes. In the treatment of periodontal diseases, the gel should be placed in the gum pockets 1-2 times a day, and also used as compresses or gently rubbed into the gums 1-2 times a day. The duration of treatment is determined depending on the clinical situation. Do not drink or eat for about half an hour after applying the gel.
Children
Do not use in children under 3 years of age.
Overdose
Theoretically, an overdose of the drug is impossible. However, if this occurs, the patient should rinse the mouth with plenty of water and, if necessary, induce vomiting. In case of hypersensitivity to salicylates, after an overdose of the gel and absorption of significant amounts of choline salicylate into the body, symptoms of the systemic effect of salicylates may occur: increased sweating, tinnitus, nausea, vomiting, dizziness, skin rashes (erythema and urticaria). Treatment is symptomatic.
Adverse reactions
A short-term burning sensation may occur at the site of application, which will later pass. Due to the presence of methylparaben and propylparaben in the composition, allergic reactions are possible.
Expiration date
3 years.
Storage conditions
Store at a temperature not exceeding 25 ° C. Do not freeze. Keep out of the reach of children.
Packaging
10 g of gel in tubes.
Vacation category
Without a prescription.
Producer
Pharmaceutical Works Jelfa SA Poland.
Location of the manufacturer and its business address
58-500 Jelenia Góra, Wincentego Pola str., 21, Poland/58-500 Jelenia Góra, Wincentego Pola str., 21, Poland
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.