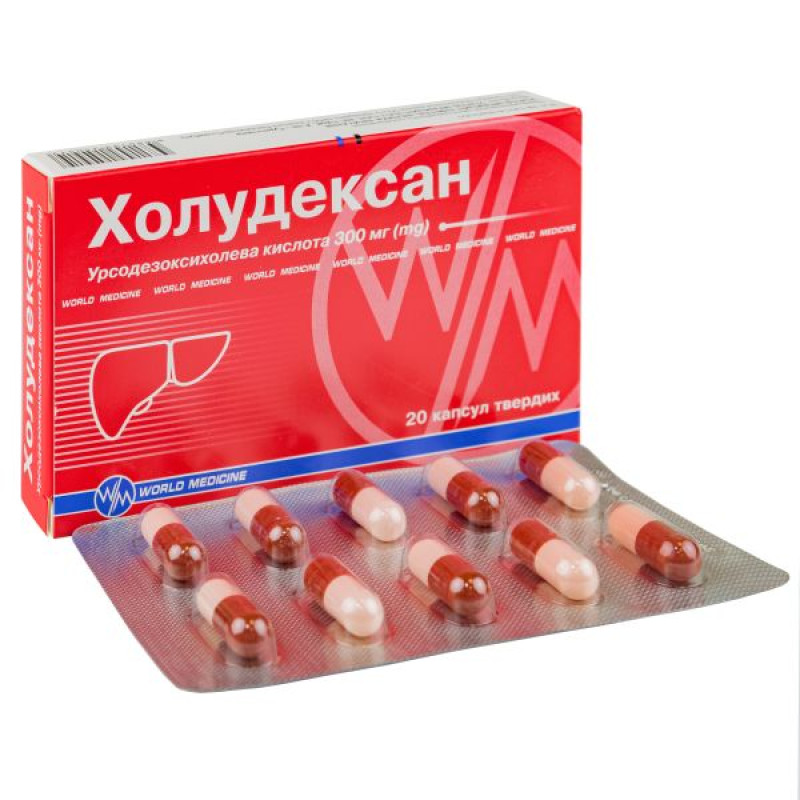
Holudexan capsules 300 mg No. 20

Instructions for use of Holudexan capsules 300 mg No. 20
Composition
active ingredient: ursodeoxycholic acid;
1 capsule contains ursodeoxycholic acid 300 mg;
excipients: corn starch, colloidal anhydrous silicon dioxide, magnesium stearate;
capsule composition: iron oxide red (E 172), titanium dioxide (E 171), gelatin.
Dosage form
The capsules are hard.
Main physicochemical properties: opaque, hard gelatin capsules, pink body, red-brown cap, containing white or almost white powder.
Pharmacotherapeutic group
Drugs used in diseases of the liver and biliary tract. Drugs used in biliary pathology. ATX code A05A A02.
Pharmacological properties
Pharmacodynamics
A small amount of ursodeoxycholic acid has been found in human bile.
After oral administration, it reduces the cholesterol saturation of bile by inhibiting its absorption in the intestine and reducing cholesterol secretion into the bile. Perhaps due to the dispersion of cholesterol and the formation of liquid crystals, the gradual dissolution of gallstones occurs.
According to current knowledge, it is believed that the effect of ursodeoxycholic acid in liver diseases and cholestasis is due to the relative replacement of lipophilic, detergent-like toxic bile acids with hydrophilic cytoprotective non-toxic ursodeoxycholic acid, improvement of the secretory capacity of hepatocytes, and immunoregulatory processes.
Use in children
Cystic fibrosis
There is information available from clinical reports regarding the long-term use of ursodeoxycholic acid (up to 10 years) in the treatment of children with hepatobiliary disorders associated with cystic fibrosis. There is evidence that the use of ursodeoxycholic acid can reduce proliferation in the bile ducts, stop the progression of histological changes, and even eliminate hepatobiliary changes if treatment is started in the early stages of cystic fibrosis. For best effectiveness, treatment with ursodeoxycholic acid should be started immediately after the diagnosis of cystic fibrosis is clarified.
Pharmacokinetics
When administered orally, ursodeoxycholic acid is rapidly absorbed in the small intestine and upper ileum by passive transport, and in the terminal ileum by active transport. The absorption rate is usually 60–80%.
After absorption, bile acid undergoes almost complete conjugation in the liver with the amino acids glycine and taurine and is then excreted in the bile. The first-pass clearance through the liver is up to 60%.
Depending on the daily dose and the underlying liver disorder or condition, the more hydrophilic ursodeoxycholic acid accumulates in the bile. At the same time, there is a relative decrease in other more lipophilic bile acids.
Under the influence of intestinal bacteria, partial degradation occurs to 7-ketolithocholic and lithocholic acids. Lithocholic acid is hepatotoxic and causes liver parenchymal damage in some animal species. In humans, only a small amount is absorbed, which is sulfated in the liver and thus detoxified before being excreted in the bile and, finally, in the feces.
The biological half-life of ursodeoxycholic acid is 3.5–5.8 days.
Indication
Diseases accompanied by impaired bile production, including cholesterol overload. Prevention of the formation and dissolution of cholesterol radio-negative gallstones in patients with a functioning gallbladder and stones in the common bile duct (residual or recurrent stones after bile duct surgery). Biliary dyspepsia. Hepatobiliary disorders in cystic fibrosis in children aged 6 to 18 years.
Contraindication
Hypersensitivity to the active substance, bile acids or any of the excipients of the medicinal product. Acute inflammation of the gallbladder or bile ducts. Obstruction of the bile ducts (obstruction of the common bile duct or cystic duct). Frequent episodes of hepatic colic. Radiopaque calcified gallstones. Violation of gallbladder contractility. Peptic ulcer of the stomach and duodenum in the active phase. Unsuccessful outcome of portoenterostomy or lack of adequate bile outflow in children with biliary atresia.
Interaction with other medicinal products and other types of interactions
Cholestyramine, colestipol, antacids containing aluminum hydroxide and/or smectite
When used simultaneously with such agents, ursodeoxycholic acid is bound in the intestine, thus preventing its absorption and reducing its effectiveness. The drug should not be used simultaneously with cholestyramine, colestipol or antacids containing aluminum hydroxide and/or smectite. If these agents are necessary, they should be taken at least 2 hours before or 2 hours after taking the drug.
When used simultaneously with ursodeoxycholic acid, the absorption of cyclosporine from the intestine may be increased. In the case of simultaneous use of these agents, the concentration of cyclosporine in the blood plasma should be checked and, if necessary, its dose should be adjusted.
Ciprofloxacin
When used simultaneously with ursodeoxycholic acid, in some cases, the absorption of ciprofloxacin may be reduced.
Rosuvastatin
In a clinical study in healthy volunteers, concomitant administration of ursodeoxycholic acid (500 mg/day) and rosuvastatin (20 mg/day) resulted in a slight increase in rosuvastatin plasma concentrations. The clinical significance of this interaction, as well as its relevance to other statins, is unknown.
Nitrendipine
Ursodeoxycholic acid has been shown to reduce the maximum plasma concentration (Cmax) and area under the curve (AUC) of the calcium antagonist nitrendipine in healthy volunteers. Careful monitoring of the patient is recommended when these agents are used concomitantly. An increase in the dose of nitrendipine may be required.
Dapsone
When used simultaneously with ursodeoxycholic acid, a weakening of the therapeutic effect of dapsone has been reported.
These data, as well as in vitro data, suggest that ursodeoxycholic acid has the potential to induce cytochrome P450 3A enzymes. However, in a well-designed interaction study with budesonide, a proven substrate of cytochrome P450 3A, no such effect was observed.
Estrogen hormones, agents for reducing cholesterol concentration in blood plasma
When used simultaneously with such agents, it is possible to increase the secretion of cholesterol by the liver and, thus, cause the formation of stones in the gallbladder, which is the opposite effect of ursodeoxycholic acid, which is used to dissolve them.
Hepatotoxic agents
The drug is not recommended for use simultaneously with such agents.
Application features
The use of the drug should be carried out under the supervision of a physician.
The basis for the use of ursodeoxycholic acid to dissolve stones is the cholesterol nature of the stones themselves, as indicated by their radiolucency.
The drug is not recommended for use in patients with frequent biliary colic, gallbladder infections, serious pancreatic disorders, or intestinal diseases that may lead to changes in the enterohepatic circulation of bile acids (ileal resection and stoma, regional ileitis).
At the beginning of long-term treatment for stone dissolution, transaminase and alkaline phosphatase levels should be monitored.
During the first three months of treatment, liver function tests (AST, ALT and GGT) should be monitored every 4 weeks, and then every 3 months. This allows for the determination of the presence or absence of response to treatment in patients with primary biliary cirrhosis (PBC) and the timely detection of potential liver dysfunction, especially in patients with advanced PBC.
Uses for dissolving cholesterol gallstones
In order to assess the progress of treatment, as well as to timely detect any signs of calcification of stones, depending on the size of the stones, visualization of the gallbladder (oral cholecystography) with examination of the obscurations in the patient's standing and supine positions (under ultrasound guidance) should be performed 6–10 months after the start of the drug. The effectiveness of the treatment should be checked by cholecystography or ultrasound examinations every 6 months.
The drug should not be used if the gallbladder cannot be visualized on X-rays or in the case of calcification of stones, impaired gallbladder contractility, or frequent hepatic colic.
Women using a medicine to dissolve gallstones should use an effective non-hormonal method of contraception, as hormonal contraceptives may increase the formation of gallstones (see sections “Interaction with other medicinal products and other types of interactions” and “Use during pregnancy or breastfeeding”).
Treatment of patients with advanced stage PBC
Decompensated liver cirrhosis has been reported very rarely during the use of ursodeoxycholic acid, which may partially regress after discontinuation of treatment.
Very rarely, at the beginning of treatment, patients with PBC may experience an increase in symptoms, for example increased itching. In such cases, the dose of the medicinal product should be reduced.
A higher probability of dissolution exists for small stones in a functioning gallbladder; bile desaturation to cholesterol is an important prognostic element of successful treatment completion, but is not decisive, since dissolution is also possible as a result of the physical process of liquid crystal formation regardless of the saturation state.
If diarrhea occurs, the dosage should be reduced. If diarrhea persists, the drug should be discontinued.
Ability to influence reaction speed when driving vehicles or other mechanisms
Use during pregnancy or breastfeeding
Pregnancy
There are insufficient data from the use of ursodeoxycholic acid in pregnant women. Animal studies have shown reproductive toxicity in early pregnancy.
The medicine should not be used during pregnancy, except in cases of urgent need.
Women of childbearing potential should only use the drug if they are using reliable contraception. It is recommended to use non-hormonal contraceptives or oral contraceptives with a low estrogen content. Patients using the drug for the dissolution of gallstones should use effective non-hormonal contraception, since hormonal oral contraceptives may increase the formation of gallstones. Pregnancy should be excluded before starting treatment.
Breastfeeding period
According to several reported cases of ursodeoxycholic acid use in breastfeeding women, its content in milk was very low, so no adverse effects should be expected in children receiving such milk.
Fertility
Animal studies have not shown any effect of ursodeoxycholic acid on fertility. There are no data on the effect on fertility in humans.
Method of administration and doses
The medicine is intended for oral use.
Long-term use to reduce the lithogenic characteristics of bile
The recommended daily dose of the drug is 5–10 mg/kg of body weight. On average, this is 300–600 mg per day (after or during meals and in the evening).
To maintain conditions conducive to stone dissolution, treatment should be continued for at least 4–6 months, and up to 12 months or longer. The drug should be continued for another 3–4 months after the disappearance of stones is confirmed by X-ray or ultrasound. However, the duration of treatment should not exceed 2 years.
Dyspeptic syndromes and supportive treatment
The recommended dose of the drug is 300 mg per day, divided into 2–3 doses (use ursodeoxycholic acid preparations in the appropriate dosage).
The dosage of the drug may be changed at the discretion of the doctor.
There are no general restrictions on the use of ursodeoxycholic acid in children, but ursodeoxycholic acid capsules should not be used in children weighing less than 47 kg. If the child weighs less than 47 kg and/or has difficulty swallowing, it is recommended to use ursodeoxycholic acid in another dosage form, such as a suspension.
Cystic fibrosis
Children aged 6 to 18 years
The recommended daily dose of the drug is 20 mg/kg body weight, divided into 2-3 doses. If necessary, the dose can be increased to 30 mg/kg body weight.
Children
The drug can be used in children aged 6 to 18 years for hepatobiliary disorders in cystic fibrosis.
Overdose
In case of an overdose of ursodeoxycholic acid, diarrhea is possible. In general, other symptoms of overdose are unlikely, since with increasing doses, the absorption of ursodeoxycholic acid decreases and most of the administered dose will be excreted in the feces. Cases of overdose of more than 4 g/day have not been described (this dose is well tolerated).
In case of overdose, no specific measures are required; the effects of diarrhea should be treated symptomatically, with restoration of fluid and electrolyte balance.
In case of accidental oral ingestion of a very high dose of ursodeoxycholic acid, it is recommended to take the usual precautions recommended for poisoning and to take cholestyramine, taking into account its ability to form bile acid chelates.
Additional information regarding special patient groups
Long-term therapy with high doses of ursodeoxycholic acid (28–30 mg/kg/day) in patients with primary sclerosing cholangitis (off-label use) was associated with a higher incidence of serious adverse events.
Adverse reactions
Adverse reactions are classified according to frequency: very common (>1/10); common (≥1/100, <1/10); uncommon (≥1/1000, <1/100); rare (≥1/10000, <1/1000); very rare (<1/10000); frequency unknown (frequency cannot be estimated from the available data).
From the digestive tract
Cases of pasty stools or diarrhea have been reported during treatment with ursodeoxycholic acid.
Very rarely, severe abdominal pain localized in the right hypochondrium was noted during the treatment of primary biliary cirrhosis.
Liver and gallbladder
Very rarely, calcification of gallstones is possible during treatment with ursodeoxycholic acid.
During the treatment of advanced stages of PBC, decompensation of liver cirrhosis is very rarely observed, which partially regressed after discontinuation of treatment.
On the part of the immune system
Very rarely, allergic reactions are possible, including rash and urticaria.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions that occur after the marketing authorisation of a medicinal product is very important. This allows the benefit/risk balance of the medicinal product to be continuously monitored. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system.
Expiration date
3 years.
Storage conditions
Store at a temperature not exceeding 25 °C out of the reach of children.
Packaging
10 capsules in a blister, 2 blisters in a cardboard box.
Vacation category
According to the recipe.
Producer
WORLD MEDICINE ILACH SAN. VE TIJ. A. Sh.
Location of the manufacturer and its business address
15 Temmuz Mahalleshi Cami Yolu Caddesi No. 50 Guneshli Bagcilar/Istanbul, Turkey.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.