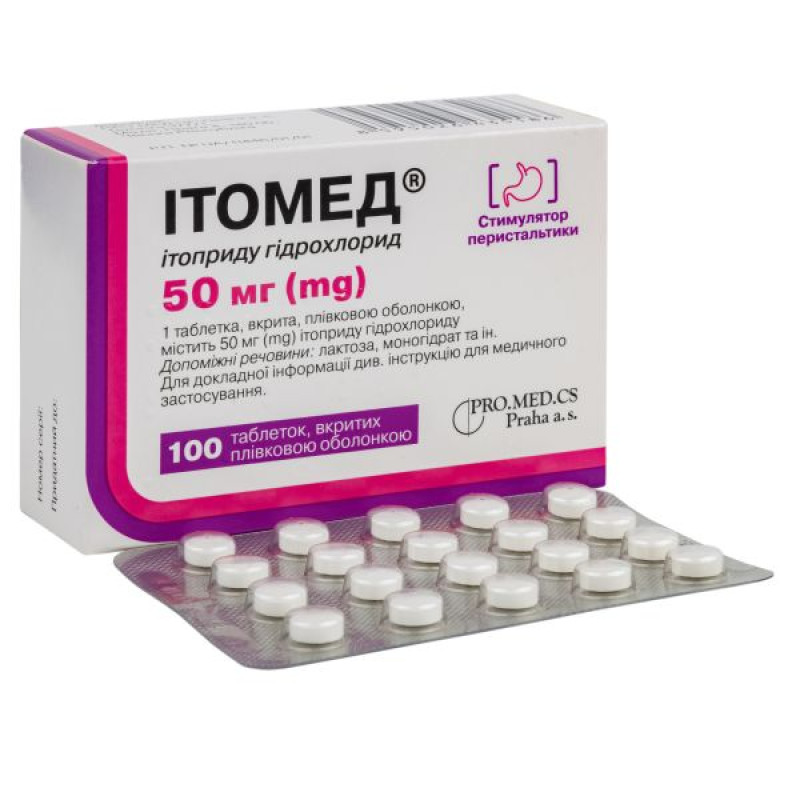
Itomed film-coated tablets 50 mg blister No. 100
Instructions for Itomed film-coated tablets 50 mg blister No. 100
Composition
active ingredient: itopride hydrochloride;
1 tablet contains 50 mg of itopride hydrochloride;
excipients: lactose monohydrate; pregelatinized starch; croscarmellose sodium; colloidal anhydrous silica; magnesium stearate; Opadry II White 85F 18422 (titanium dioxide (E 171), polyvinyl alcohol, talc, polyethylene glycol).
Dosage form
Film-coated tablets.
Main physicochemical properties: white or almost white film-coated tablets, biconvex, with a score on one side, with a diameter of about 7 mm.
Pharmacotherapeutic group
Drugs used in functional disorders of the digestive tract. Peristalsis stimulants. ATX code A03F A.
Pharmacological properties
Pharmacodynamics
Itopride hydrochloride activates propulsive motility of the gastrointestinal tract due to antagonism with dopamine D2 receptors and inhibitory activity of acetylcholinesterase. Itopride hydrochloride activates the release of acetylcholine and inhibits its breakdown.
Itopride hydrochloride also has an antiemetic effect due to its interaction with
D2 receptors localized in the chemoreceptor trigger zone, as demonstrated by dose-dependent inhibition of apomorphine-induced vomiting in animals.
The action of itopride hydrochloride is highly specific for the upper gastrointestinal tract.
Itopride hydrochloride does not affect serum gastrin levels.
Itopride hydrochloride accelerates the evacuation of gastric contents, improves gastroduodenal coordination, and stimulates the passage of intestinal contents.
Pharmacokinetics
Absorption
Itopride hydrochloride is rapidly and almost completely absorbed from the gastrointestinal tract. Its relative bioavailability is 60%, which is associated with metabolism during the "first pass" through the liver (presystemic metabolism). Food intake does not affect the bioavailability of the drug. The maximum concentration of the drug Cmax in the blood plasma is achieved within 30-45 minutes after taking 50 mg of itopride hydrochloride and is 0.28 μg / ml. It does not penetrate the blood-brain barrier and into the cells of the myocardial conduction system.
With continued use of the drug in doses from 50 to 200 mg three times a day for 7 days, the pharmacokinetics of itopride hydrochloride and its metabolites were linear with minimal drug accumulation.
Distribution
Approximately 96% of itopride hydrochloride is bound to plasma proteins (mainly albumin). Binding to alpha-1-acid glycoprotein is less than 15%.
Animal studies have shown that itopride hydrochloride is widely distributed to various tissues (volume of distribution 6.1 l/kg), except the central nervous system; high drug concentrations are achieved in the kidneys, small intestine, liver, adrenal glands and stomach. Penetration of the drug into the CNS is minimal. Itopride hydrochloride is excreted in breast milk of animals.
Metabolism
In humans, itopride hydrochloride undergoes extensive metabolism in the liver. Three metabolites of the drug have been identified, only one of which exhibits negligible activity that is not pharmacologically significant (approximately 2-3% of the pharmacological activity of itopride hydrochloride). The main metabolite in humans is the N-oxide, which is formed as a result of oxidation of the quaternary amino-N-dimethyl group.
Itopride hydrochloride is metabolized by flavin-dependent monooxygenase (FMO). The number and efficiency of FMO isoenzymes in humans may vary depending on genetic polymorphisms, which can sometimes lead to a rare autosomal recessive disorder known as trimethylaminuria (fish odor syndrome). The elimination half-life T½ of itopride hydrochloride may be longer in patients with trimethylaminuria.
According to in vivo pharmacokinetic studies, itopride hydrochloride does not inhibit or induce CYP2C19 and CYP2E1. The use of itopride hydrochloride did not affect the content of CYP enzymes or the activity of uridine diphosphate glucuronosyl transferase.
Breeding
Itopride hydrochloride and its metabolites are excreted mainly in the urine. In healthy volunteers, after a single oral dose of the drug, the excretion of itopride and its N-oxide metabolite was 3.7% and 75.4%, respectively.
The half-life of itopride hydrochloride is approximately 6 hours.
Indication
Relief of gastrointestinal symptoms of functional non-ulcer dyspepsia (chronic gastritis), namely:
bloating; feeling of rapid satiety; pain and discomfort in the upper abdomen; heartburn; nausea; vomiting; anorexia.
Contraindication
Hypersensitivity to the active substance or to any component of the drug.
Conditions in which increased contractile activity of the gastrointestinal tract may be harmful, such as gastrointestinal bleeding, mechanical obstruction or perforation.
Interaction with other medicinal products and other types of interactions
No changes in protein binding were observed when it was co-administered with warfarin, diazepam, diclofenac sodium, ticlopidine hydrochloride, nifedipine and nicardipine hydrochloride. Since itopride hydrochloride increases gastric motility, it may affect the absorption of other drugs when co-administered. Particular caution should be exercised when using drugs with a low therapeutic index, sustained-release dosage forms or enteric-coated drugs.
Antiulcer drugs such as cimetidine, ranitidine, teprenone and cetraxate do not affect the prokinetic effect of itopride hydrochloride.
Anticholinergic drugs may reduce the therapeutic effect of itopride hydrochloride.
Application features
Itopride hydrochloride enhances the action of acetylcholine and may cause cholinergic side effects. Long-term data are not available.
Elderly people, given their decreased liver and kidney function and concomitant diseases or concomitant therapy with other drugs, should be cautious when using itopride due to the possible more frequent development of adverse reactions.
If a dose is missed, it should be taken as soon as possible; do not use if it is time for the next dose; do not double the dose.
The drug contains lactose, therefore patients with rare hereditary forms of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption syndrome should not use the drug.
Ability to influence reaction speed when driving vehicles or other mechanisms
There is no information on the possible effect on reaction speed, but when deciding whether to drive or operate other mechanisms, the possibility of dizziness should be taken into account.
Method of administration and doses
Adults should take 1 tablet (50 mg) 3 times a day before meals, without chewing and with sufficient water.
The recommended daily dose is 150 mg. This dose may be reduced based on clinical symptoms and the patient's age (see "Special instructions").
Patients with impaired liver and kidney function should use the drug under proper medical supervision; if necessary, the dose should be reduced or therapy discontinued.
The duration of treatment is determined by the doctor. During clinical studies, the duration of use of itopride hydrochloride was up to 8 weeks.
Children
The drug should not be used in children.
Overdose
No cases of overdose have been reported.
Treatment: In case of excessive overdose, the usual measures of gastric lavage and symptomatic treatment should be taken.
Adverse reactions reported during clinical trials.
During clinical trials (phases I-III), itopride hydrochloride was well tolerated, and no serious adverse reactions were reported. A total of 19 adverse reactions were reported, occurring in 14 of 572 patients, or 2.4%. The majority of these adverse reactions, occurring in more than one patient, were: diarrhea (0.7%), headache (0.3%), and abdominal pain (0.3%).
Laboratory abnormalities observed during clinical trials: decreased white blood cell count (leukopenia) (0.7%), increased prolactin levels (0.3%).
Adverse reactions reported during post-marketing surveillance and in ongoing clinical trials in patients treated with itopride hydrochloride:
From the blood and lymphatic system: leukopenia, thrombocytopenia.
On the part of the digestive system: diarrhea, constipation, abdominal pain, increased salivation, nausea.
Liver and biliary tract disorders: jaundice.
Immune system disorders: anaphylactoid reaction.
On the part of the endocrine system: possible increase in blood prolactin levels, gynecomastia or galactorrhea. In the event that, for example, galactorrhea or gynecomastia occur, it is necessary to temporarily stop or completely cancel the drug.
From the nervous system: dizziness, headache, insomnia, tremor.
Renal and urinary disorders: increased blood creatinine levels; increased urine creatinine levels.
Skin and subcutaneous tissue disorders: allergic reactions (rash, redness, itching).
Others: increased fatigue, irritability.
Laboratory tests: increased levels of AST, ALT, GGT, alkaline phosphatase, bilirubin.
Expiration date
5 years.
Storage conditions
Keep out of the reach of children. No special storage conditions required.
Packaging
20 tablets in a blister, 5 blisters in a cardboard box.
Vacation category
According to the recipe.
Producer
PRO.MED.CS Prague as (PRO.MED.CS Prahа as).
Location of the manufacturer and its business address
Telčska 377/1, Michle, Prague 4, 140 00, Czech Republic.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.