Kratal tablets blister pack No. 20
Instructions for Kratal tablets blister No. 20
Composition
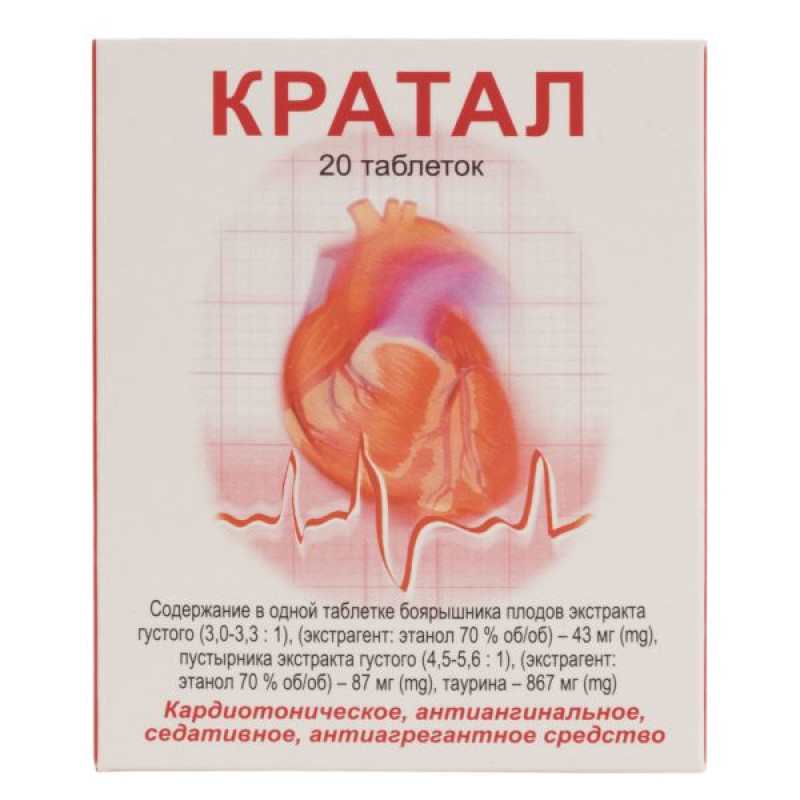
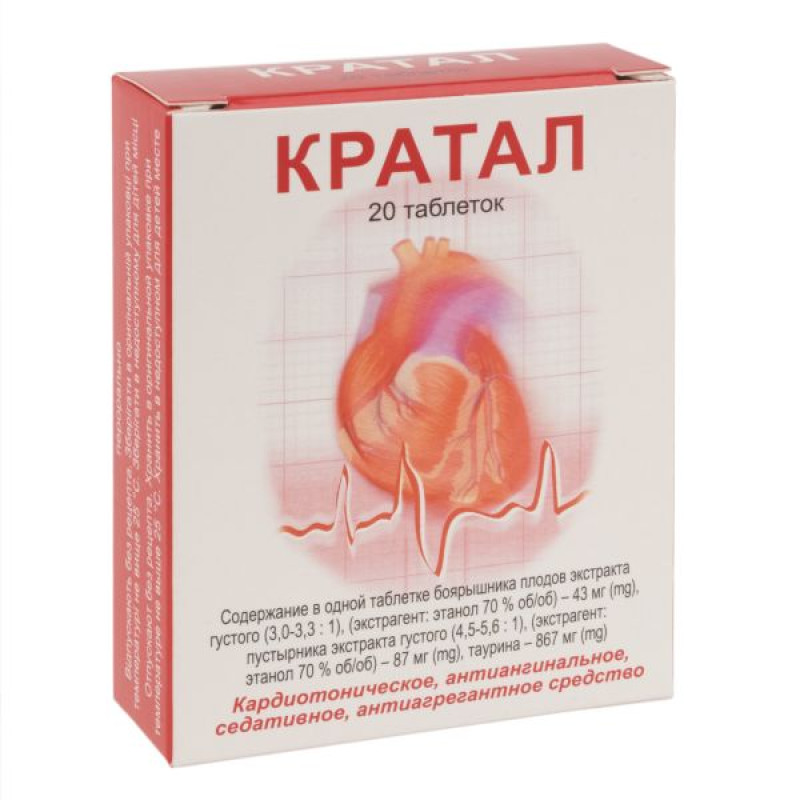
active ingredients: 1 tablet contains hawthorn fruit extract thick (Crataegiae fructus extractum spissum) (3.0-3.3:1), (extractant: ethanol 70% v/v), calculated on dry matter – 43 mg, stinging nettle extract thick (Leonuriae herba extractum spissum) (4.5-5.6:1), (extractant: ethanol 70% v/v), calculated on dry matter – 87 mg, taurine, calculated on 100% dry matter – 867 mg;
excipients: microcrystalline cellulose, croscarmellose sodium, magnesium stearate, colloidal anhydrous silicon dioxide.
Dosage form
Pills.
Main physicochemical properties: oval tablets from light gray or light brown to dark brown in color, with inclusions, with a biconvex surface.
Pharmacotherapeutic group
Combined cardiological agents. ATX code C01E X.
Pharmacological properties
Pharmacodynamics
Kratal has a mild cardiotonic, antianginal, antioxidant, antiarrhythmic, antihypoxic, antiplatelet and antiatherogenic effect; inhibits the renin-angiotensin and kallikrein-kinin systems, lipid peroxidation processes, has a positive effect on the production of cyclic adenosine monophosphate (cAMP). Improves blood supply and functional state of the myocardium, increases "coronary reserve", improves contractile and pumping function of the heart muscle, reduces blood pressure and normalizes heart rate.
Kratal reduces the manifestations of digitalis intoxication. Increases working capacity, improves mood, has a calming, neuroprotective effect (eliminates somatonegative disorders - irritability, mood swings).
Pharmacokinetics
The components of the drug are almost completely absorbed from the digestive tract. The time for which the maximum concentration of taurine and other components of the drug in the blood is reached is 2 hours. Kratal is evenly distributed in tissues and body fluids. The main amount of it is excreted in the urine, the half-life is 6-8 hours.
Indication
Neurocirculatory dystonia; as part of combination therapy for: chronic ischemic heart disease; post-radiation syndrome.
Contraindication
Hypersensitivity to the components of the drug; pronounced bradycardia and arterial hypotension.
Interaction with other medicinal products and other types of interactions
Potentiates the antianginal effects of nitrates, β-blockers, calcium antagonists, antihypoxants, neuroprotectors, cardiac glycosides. Reduces the manifestations of digitalis intoxication. The drug should not be used together with class III antiarrhythmics, cisapride.
Application features
Do not exceed the recommended doses of the drug.
If symptoms persist or worsen during treatment with the drug, you should consult a doctor.
If you experience swelling of your ankles or feet, pain in the heart area that may spread to the left arm, upper abdomen, or neck, or shortness of breath, seek IMMEDIATE medical attention.
Ability to influence reaction speed when driving vehicles or other mechanisms
During the period of treatment, patients taking Kratal should refrain from driving vehicles and potentially dangerous mechanisms.
Use during pregnancy or breastfeeding
There is currently no data on the use of the drug during pregnancy or breastfeeding.
The drug is contraindicated for use during pregnancy.
If it is necessary to use the drug in breastfeeding women, breastfeeding should be discontinued for the period of treatment.
Method of administration and doses
The duration of treatment and dose are determined by the doctor individually. The drug is administered orally, 1-2 tablets 3 times a day before meals. The course of treatment is 3-4 weeks.
Children
There is no experience in using the drug to treat children.
Overdose
Symptoms: hypersensitivity reactions, allergic reactions, dyspeptic reactions, general weakness, feeling of fatigue, dizziness, drowsiness, arterial hypotension, bradycardia.
Treatment: discontinuation of the drug and symptomatic therapy.
Adverse reactions
Possible manifestations of hypersensitivity, allergic reactions (including hyperemia, rashes, itching, swelling of the skin, urticaria), dyspeptic phenomena, general weakness, increased fatigue, dizziness, drowsiness, arterial hypotension, bradycardia.
If any adverse reactions occur, the patient should consult a doctor.
Expiration date
3 years.
Do not use after the expiry date stated on the packaging.
Storage conditions
In the original packaging at a temperature not exceeding 25 °C.
Keep out of reach of children.
Packaging
10 tablets in a blister, 2 blisters in a pack.
Vacation category
Without a prescription.
Producer
Location of the manufacturer and its business address
Ukraine, 03134, Kyiv, Myru St., 17.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.