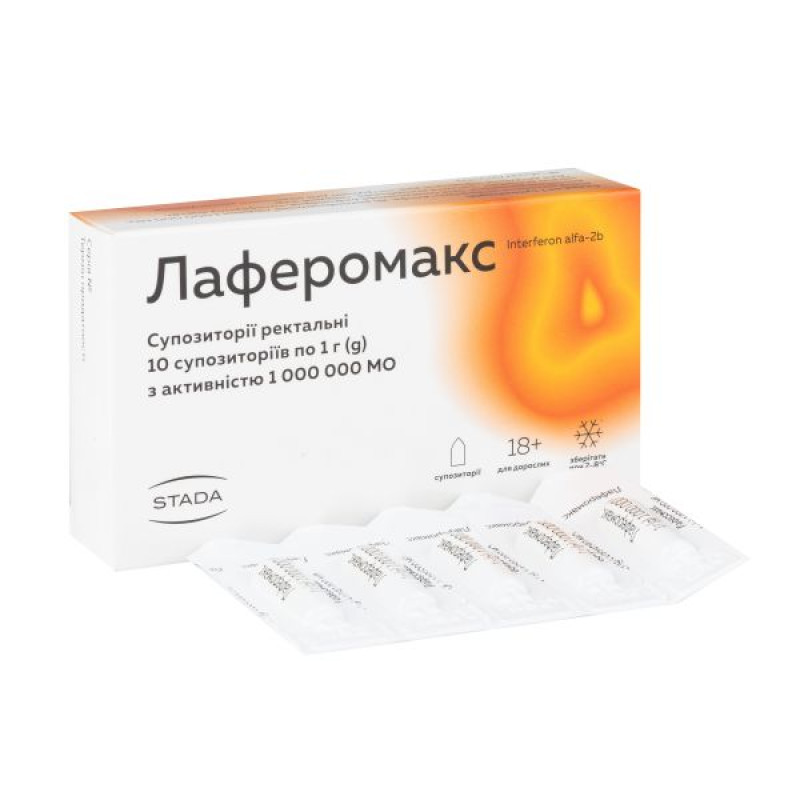
Laferomax suppositories 1000000 IU No. 10

Instructions for Laferomax suppositories 1000000 IU No. 10
Composition
active ingredient: interferon alfa-2b
1 suppository contains: recombinant human interferon alpha-2b – 1,000,000 IU or 3,000,000 IU;
excipients: tocopherol acetate 5% oily solution, ascorbic acid, solid fat.
Dosage form
Suppositories.
Main physicochemical properties: spherical suppositories of yellowish-white color with a homogeneous consistency.
Pharmacotherapeutic group
Immunostimulants. Interferon alpha-2b.
ATX code L03A B05.
Pharmacological properties
Interferon alpha-2b recombinant has a pronounced antiviral, antiproliferative and immunomodulatory effect. The complex composition of Laferomax causes a number of new effects: in combination with tocopherol acetate and ascorbic acid, the antiviral activity of interferon alpha-2b recombinant increases by 10-14 times, its immunomodulatory effect on T- and B-lymphocytes is enhanced, the content of immunoglobulin E is normalized. Antibodies that neutralize the antiviral activity of interferon-alpha-2b recombinant are not formed, even when it is used for 2 years, the functioning of the endogenous system is normalized.
Indication
Adult:
- for papillomavirus infections (vulgar warts, condyloma acuminatum);
- for urogenital mixed infections that are sexually transmitted;
- in precancerous diseases of the cervix.
Contraindication
Hypersensitivity to the components of the drug; the presence of thyroid dysfunction in the patient; the presence of severe visceral disorders in patients with Kaposi's sarcoma; severe cardiovascular diseases; psoriasis; severe liver and/or kidney dysfunction; epilepsy and other CNS diseases (including functional); chronic hepatitis against the background of progressive or decompensated liver cirrhosis; chronic hepatitis in patients receiving or recently receiving immunosuppressant therapy (except for a short course of corticosteroid therapy); autoimmune hepatitis or other autoimmune diseases in history. Suppression of the myeloid germ of hematopoiesis.
Interaction with other medicinal products and other types of interactions
The drug should be used with caution simultaneously with opioid drugs, analgesics, hypnotics and sedatives (potentially causing a myelosuppressive effect).
When used simultaneously with drugs that are metabolized by oxidation (including xanthine derivatives - aminophylline and theophylline), the possibility of the effect of Laferomax on oxidative metabolic processes should be taken into account. The concentration of theophylline in the blood serum should be monitored and, if necessary, the dosage regimen should be adjusted.
When using the drug in combination with chemotherapeutic drugs (cytarabine, doxorubicin, teniposide, cyclophosphamide), the risk of developing life-threatening toxic effects (their severity and duration) increases.
When used simultaneously with zidovudine, the risk of developing neutropenia increases.
Application features
Treatment with Laferomax should be carried out under the supervision of a physician.
Before prescribing the drug for a long time, it is recommended to study the function of the thyroid gland. The drug should be started on condition that the level of thyroid-stimulating hormone (TSH) is within normal limits. If any changes in the level of thyroid-stimulating hormone are detected, appropriate therapy should be carried out and therapy with Laferomax should be started on condition that the content of thyroid-stimulating hormone can be maintained at a normal level. During treatment, it is also advisable to monitor the level of thyroid-stimulating hormone.
After discontinuation of therapy, thyroid function, which is impaired as a result of drug administration, does not recover.
All patients are recommended to undergo a comprehensive peripheral blood test before starting and regularly during treatment, with mandatory qualitative and quantitative blood tests, as well as a biochemical blood test, including determination of electrolytes, calcium, liver enzymes, and creatinine.
In myeloma disease, periodic monitoring of kidney function is necessary.
It is recommended that serum albumin levels and prothrombin time be carefully monitored in all patients receiving the drug.
The drug should be prescribed with caution in patients with a history of diseases such as diabetes mellitus with episodes of ketoacidosis and chronic obstructive pulmonary disease, with blood clotting disorders (including pulmonary artery thrombophlebitis), and with severe myelosuppression.
During treatment with the drug, it is necessary to ensure adequate hydration of the body; in case of fever, other causes of its occurrence should be excluded.
It is recommended to use the drug against the background of antihistamine and antipyretic therapy.
If an immediate-type hypersensitivity reaction develops (urticaria, angioedema, bronchospasm, anaphylaxis), the drug should be immediately discontinued and appropriate measures taken.
Discontinue use of the drug in cases of: prolonged blood clotting time (in patients with chronic hepatitis), manifestations of pulmonary syndrome and radiological detection of infiltrate or impaired lung function, appearance or increase in visual impairment, thyroid dysfunction (deviation from the normal level of TSH), decreased serum albumin levels and decreased prothrombin time.
The use of the drug after the expiration date is unacceptable. The drug is not subject to repeated quality control and extension of the shelf life after its expiration.
Use during pregnancy or breastfeeding
There are no data on the use of the drug during pregnancy and breastfeeding.
Ability to influence reaction speed when driving vehicles or other mechanisms
Some adverse CNS effects caused by the use of the drug may affect patients' ability to drive vehicles and operate potentially dangerous machinery.
Method of administration and doses
Adult:
- for papillomavirus infections (genital warts, condyloma acuminatum) the drug is prescribed at 1,500,000 IU 2 times a day with a 12-hour break rectally. The course of treatment is 10 days. The treatment is carried out by monitoring the patient's condition using cytological, bacteriological examination and observing the manifestations of clinical symptoms;
- for mixed urogenital infections transmitted sexually, and for bacterial, viral and mixed infections, the drug is prescribed at 1,000,000 - 1,500,000 IU 2 times a day with a 12-hour break rectally. The course of treatment is 10 days. The treatment is carried out by monitoring the patient's condition using cytological, bacteriological examination and observing the manifestations of clinical symptoms. The treatment is carried out for both sexual partners;
- for precancerous diseases of the cervix, 1,500,000 IU 2 times a day with a 12-hour break rectally. The course of treatment is 10 days. Further treatment is determined by clinical and laboratory indicators.
Children
Not used in pediatric practice.
Overdose
To date, no cases of overdose with Laferomax have been described. However, as with any drug overdose, symptomatic therapy with monitoring of vital organ functions and careful observation of the patient's condition is recommended.
Adverse reactions
All adverse reactions associated with the use of Laferomax are minor or moderate in severity. They usually disappear after the end of treatment.
General disorders. When administering Laferomax, flu-like symptoms are possible: chills, fever, fatigue, lethargy, as well as headache, muscle and joint pain, sweating; rarely - vomiting, dizziness, hot flashes. Hypersensitivity reactions to the drug are possible.
Disorders of the hematopoietic system: with prolonged use, leukopenia, thrombocytopenia, anemia, and nosebleeds are possible.
Gastrointestinal and hepatic disorders: increased ALT and AST levels, increased LF levels, loss of appetite. Liver function disorders.
Endocrine disorders: thyroid dysfunction.
Central and peripheral nervous system disorders: with prolonged use, dizziness, sleep disturbances, confusion, anxiety and depressive states, increased excitability, drowsiness, ataxia, paresthesias are possible.
Cardiovascular system disorders: possible arterial hypertension or hypotension; rarely - tachycardia.
Skin and subcutaneous tissue disorders: allergic reactions, including rashes (including herpetic), itching, hyperemia.
Respiratory system disorders – cough.
Other: changes at the injection site, visual disturbances, renal dysfunction, electrolyte imbalance.
Expiration date
2 years.
Storage conditions
Store in the original packaging to protect from light at a temperature of 2 ºС to 8 ºС.
Keep out of reach of children.
Packaging
3 or 5 suppositories in a contour blister pack. 1 contour blister pack with 3 or 5 suppositories or 2 contour blister packs with 5 suppositories in a cardboard box.
Vacation category
According to the recipe.
Producer
PrJSC "BIOPHARMA", Ukraine;
LLC "FZ "BIOPHARMA", Ukraine.
Location of the manufacturer and address of its place of business
Ukraine, 03680, Kyiv, M. Amosova St., 9;
Ukraine, 09100, Kyiv region, Bila Tserkva, Kyivska st., 37.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.