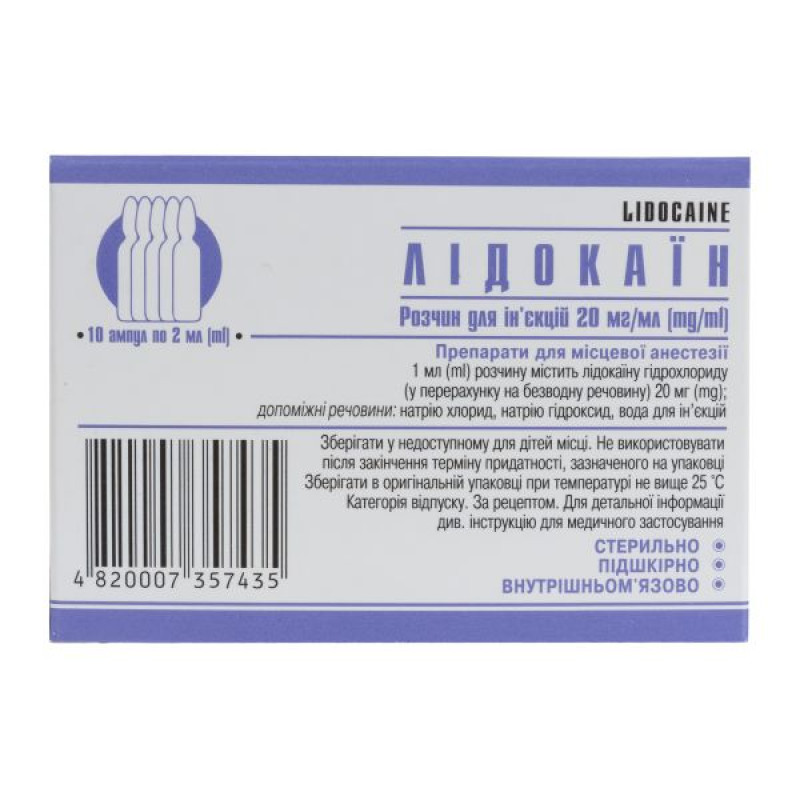
Lidocaine solution for injection 20 mg/ml ampoule 2 ml No. 10
Instructions Lidocaine solution for injection 20 mg/ml ampoule 2 ml No. 10
Composition
active ingredient: lidocaine;
1 ml of liquid contains lidocaine hydrochloride (calculated as anhydrous substance) 20 mg;
excipients: sodium chloride, sodium hydroxide, water for injections.
Dosage form
Solution for injection.
Main physicochemical properties: clear colorless or slightly colored solution, practically free from particles.
Pharmacotherapeutic group
Local anesthetics. Lidocaine. ATX code N01B B02.
Pharmacological properties
Pharmacodynamics.
Derivative of acetanilide. Local anesthetic agent that produces terminal, infiltration, and conduction anesthesia. The relative toxicity of lidocaine hydrochloride depends on the concentration of the solution. In low concentrations (0.5%) it does not significantly differ in toxicity from novocaine, with increasing concentration (1% and 2%) the toxicity increases (40–50%).
Pharmacokinetics.
When applied topically to mucous membranes, lidocaine is absorbed to varying degrees, depending on the dose and site of application (maximum concentration (Cmax) is reached after 10–20 minutes); absorption is affected by the rate of perfusion into the mucous membrane. When administered intramuscularly, Cmax is reached after 5–15 minutes. Binding to blood plasma proteins is 60–80% (depending on the dose). It easily passes through histohematological barriers, including the blood-brain barrier. First, it enters tissues with good blood supply (heart, lungs, brain, liver, spleen), then into adipose and muscle tissue. It penetrates the placenta, and 40–55% of the concentration of the drug applied to the mother is found in the newborn.
It is metabolized by 90% in the liver by oxidative N-dealkylation to form active metabolites: monoethylglycinexylidine and glycinexylidine, which have a half-life (T½) of 2 hours and 10 hours, respectively. It has a "first-pass" effect.
In case of impaired liver function, T½ may increase more than 2-fold. 5–20% is excreted unchanged in the urine.
Indication
Local anesthesia (terminal, infiltration, conduction) in surgery, ophthalmology, dentistry, otorhinolaryngology; blockade of peripheral nerves and nerve plexuses for various pain syndromes.
Contraindication
Hypersensitivity to the components of the drug or to other amide local anesthetics, history of epileptiform seizures after the use of lidocaine, severe bradycardia, severe arterial hypotension, cardiogenic shock, severe forms of chronic heart failure (II-III degree), sick sinus syndrome, Wolff-Parkinson-White syndrome, Adams-Stokes syndrome, atrioventricular (AV) block of II and III degree, hypovolemia, severe liver/kidney dysfunction, porphyria, myasthenia, retrobulbar administration in patients with glaucoma.
Interaction with other medicinal products and other types of interactions
When combined with lidocaine with drugs such as chlorpromazine, pethidine, bupivacaine, quinidine, disopyramide, amitriptyline, imipramine, nortriptyline, the concentration of lidocaine in the blood plasma increases due to a decrease in hepatic metabolism.
Antiarrhythmic drugs (including amiodarone, verapamil, quinidine, disopyramide, ajmalin) and class IA antiarrhythmic drugs (including quinidine, procainamide, disopyramide) or some antipsychotics (including olanzapine, quetiapine) or HT3 antagonists (including tropisetron, dolasetron) - the cardiodepressive effect is enhanced (QT interval prolongation occurs, in rare cases AV block or ventricular fibrillation may develop); simultaneous use with amiodarone may lead to the development of seizures.
Novocaine, novocainamide, procainamide – possible excitation of the central nervous system, delirium, hallucinations.
Curare-like drugs - muscle relaxation increases (paralysis of respiratory muscles is possible).
Ethanol enhances the respiratory depressant effect of lidocaine.
Vasoconstrictors (epinephrine, methoxamine, phenylephrine) help slow the absorption of lidocaine and prolong its effect.
Cimetidine reduces the hepatic clearance of lidocaine (reduced metabolism due to inhibition of microsomal oxidation), increases its concentration and the risk of developing toxic effects.
Guanadrel, guanethidine, mecamylamine, trimetaphan – when used in combination for spinal and epidural anesthesia, the risk of severe hypotension and bradycardia increases.
β-blockers slow down the metabolism of lidocaine in the liver, enhance the effects of lidocaine (including toxic ones) and increase the risk of developing bradycardia and arterial hypotension. When β-blockers and lidocaine are used simultaneously, the dose of the latter should be reduced.
Cardiac glycosides – the cardiotonic effect of cardiac glycosides is weakened.
Digitalis glycosides – against the background of lidocaine intoxication, it can increase the severity of AV blockade.
Sleeping pills or sedatives - possible increase in the CNS depressant effect of sleeping pills and sedatives.
Monoamine oxidase inhibitors (MAO) (furazolidone, procarbazine, selegiline) - the risk of arterial hypotension increases and the local anesthetic effect of the latter is prolonged. Lidocaine should not be used parenterally during treatment with MAO inhibitors.Anticoagulants (including ardeparin, dalteparin, danaparoid, enoxaparin, heparin, warfarin, etc.) increase the risk of bleeding.
Anesthetics – the depressing effect on the respiratory center of anesthetics (hexobarbital, sodium thiopental intravenously) is enhanced.
Polymyxin B – respiratory function monitoring is necessary.
Rifampicin – a decrease in the concentration of the latter in the blood is possible.
Propafenone – possible increase in duration and severity of CNS side effects.
Prenylamine – increases the risk of developing torsades de pointes.
Anticonvulsants, barbiturates (phenobarbital) - possible acceleration of lidocaine metabolism in the liver, decrease in blood concentration, and increased cardiodepressive effect.
Isadrin, glucagon – increases the clearance of lidocaine.
Norepinephrine, mexiletine – decreases lidocaine clearance (increases toxicity); decreases hepatic blood flow.
Acetazolamide, thiazide and loop diuretics reduce the effect of lidocaine by causing hypokalemia.
Midazolam – increases the concentration of lidocaine in blood plasma.
Drugs that cause blockade of neuromuscular transmission - the effect of these drugs is enhanced, as they reduce the conductivity of nerve impulses.
Application features
Lidocaine can only be administered by medical professionals.
When treating the injection site with disinfectant solutions containing heavy metals, the risk of developing local reactions such as soreness and swelling increases.
During the use of lidocaine, ECG monitoring is mandatory. In case of sinus node dysfunction, prolongation of the PQ interval, QRS expansion, or the development of a new arrhythmia, the dose should be reduced/the drug should be discontinued. In case of severe bradycardia, 0.5–1 mg of atropine should be administered intravenously. In case of arterial hypotension, if necessary, sympathomimetics and/or β-receptor agonists should be administered intravenously.
Before using lidocaine for heart disease (hypokalemia reduces the effectiveness of lidocaine), it is necessary to normalize the level of potassium in the blood.
Before administering high doses of lidocaine, it is recommended to administer barbiturates.
When performing planned subarachnoid anesthesia, MAO inhibitors must be discontinued at least 10 days before anesthesia.
Care should be taken to avoid accidental intravasal (especially when performing local anesthesia in areas containing many blood vessels) or subdural administration of the drug. It is necessary to establish close monitoring for systemic toxic effects of the drug on the cardiovascular and central nervous systems (since the doses prescribed for epidural anesthesia are always higher than for subdural). When administering to vascularized tissues, an aspiration test is recommended.
Extreme caution should be exercised when performing paraspinal anesthesia in patients with neurological diseases, spinal deformity, and septicemia.
Smaller doses of the drug should be administered to the head and neck area, including retrobulbar and dental administration, as well as use for stellate ganglion blockade, since systemic toxic effects of the drug may penetrate the cerebral circulation through retrograde flow.
Extreme caution should be exercised with retrobulbar administration, as severe side effects are possible: collapse, shortness of breath, seizures, and reversible blindness.
Since lidocaine has a pronounced antiarrhythmic effect and can itself act as an arrhythmogenic factor that can cause the development of arrhythmia, before administering the drug, it is necessary to collect a history for signs of arrhythmia and use the drug with caution in individuals with complaints of arrhythmias in the past.
It is used with caution and in lower doses in patients with moderate heart failure, moderate arterial hypotension, incomplete AV block, intraventricular conduction disorders, moderate liver and kidney dysfunction (creatinine clearance not less than 10 ml/min), respiratory dysfunction, epilepsy, after heart surgery, with a genetic predisposition to malignant hyperthermia, debilitated patients and elderly patients.
Intramuscular administration of lidocaine may increase creatinine concentration, which may lead to an error in the diagnosis of acute myocardial infarction.
Paracervical block can cause fetal bradycardia/tachycardia, so careful monitoring of fetal heart rates is necessary.
The effect of local anesthetics is reduced if the injection was made into an inflamed or infected area.
Lidocaine, solution for injection, may have a porphyrinogenic effect, so it should be prescribed only for vital indications.
This medicinal product contains 6 mg/ml sodium chloride, i.e. essentially sodium-free.
Use during pregnancy or breastfeeding
The drug is contraindicated during pregnancy.
If necessary, use of the drug should be discontinued.
Ability to influence reaction speed when driving vehicles or other mechanisms
After using the drug, it is not recommended to engage in activities that require speed of psychomotor reactions.
Method of administration and doses
The drug is used by injection (subcutaneously, intramuscularly) and topically on mucous membranes. Intravascular administration of the drug should be avoided.
For terminal anesthesia, the mucous membranes of adults are lubricated with the drug at a dose of up to 2 mg/kg of lidocaine, the duration of anesthesia is 15–30 minutes. The maximum dose of the drug for adults is 4.5 mg/kg of body weight, the maximum total dose is 300 mg.
For conduction anesthesia (including for anesthesia of the brachial and sacral plexuses) 5–10 ml (100–200 mg of lidocaine) of the drug is administered, for anesthesia of the fingers of the extremities, nose, ears – 2–3 ml (40–60 mg) (2%) of lidocaine solution for injection. The maximum dose of the drug for adults is 10 ml (200 mg).
For anesthesia in ophthalmology, 2 drops of the drug are instilled into the conjunctival sac 2–3 times with an interval of 30–60 seconds immediately before the examination or surgical intervention.
For children aged 12 years and older, the total dose of lidocaine for all types of peripheral anesthesia should not exceed 3 mg/kg of body weight.
For all types of injection anesthesia, it is possible to combine lidocaine with epinephrine (1:50,000–1:100,000; prepared ex tempore, add 1 drop of 0.1% epinephrine solution to 5–10 ml of 2% lidocaine solution), except in cases where the systemic effect of epinephrine (adrenaline) is undesirable (hypersensitivity to epinephrine, hypertension, diabetes mellitus, glaucoma) or a short-term anesthetic effect is required. Epinephrine helps slow the absorption of lidocaine and prolongs its effect.
Children.
The drug is used in children over 12 years of age.
Overdose
Possible increase in adverse reactions.
Symptoms: psychomotor agitation, dizziness, general weakness, decreased blood pressure, tremor, visual impairment, tonic-clonic convulsions, coma, collapse, AV block, asphyxia, apnea. The first symptoms of overdose occur when the concentration of lidocaine in the blood exceeds 0.006 mg/kg, convulsions - at 0.01 mg/kg.
Treatment: discontinuation of the drug, oxygen therapy, anticonvulsants, vasoconstrictors (noradrenaline, mezaton), anticholinergics. The patient should be in a horizontal position; it is necessary to ensure access to fresh air, oxygen supply and/or artificial respiration. Symptoms from the central nervous system are corrected by the use of short-acting benzodiazepines/barbiturates. If an overdose occurs during anesthesia, a short-acting muscle relaxant should be used. Atropine (0.5–1 mg intravenously) is used to correct bradycardia and conduction disorders, and sympathomimetics in combination with β-adrenoceptor agonists are used for arterial hypotension. In case of cardiac arrest, immediate resuscitation measures are indicated. Intubation and artificial ventilation of the lungs are possible. Dialysis is ineffective in the acute phase of overdose. There is no specific antidote.
Adverse reactions
From the nervous system: excitation of the central nervous system (when used in high doses), anxiety, headache, dizziness, sleep disturbances, confusion, drowsiness, loss of consciousness, coma, impaired sensitivity, numbness of the tongue and lips (when used in dentistry), motor block, disorientation; in patients with increased sensitivity - euphoria, tremor, trismus, motor restlessness, paresthesias, convulsions.
From the blood system: methemoglobinemia.
On the part of the organs of vision: nystagmus, reversible blindness, diplopia, flashing "flies" before the eyes, photophobia, conjunctivitis.
From the auditory system: hearing impairment, tinnitus, hyperacusis.
From the cardiovascular system: when used in high doses - arrhythmia, bradycardia, slowing of cardiac conduction, transverse heart block, cardiac arrest, peripheral vasodilation, collapse, tachycardia, increase/decrease in blood pressure, heart pain.
On the part of the digestive system: nausea, vomiting.
Respiratory system: shortness of breath, rhinitis, respiratory depression or arrest.
Allergic reactions: skin rashes, urticaria, itching, generalized exfoliative dermatitis, angioedema, anaphylactic reactions (including anaphylactic shock), edema.
Others: feeling hot, cold or numb in the extremities, swelling, weakness, malignant hyperthermia.
Local reactions: a slight burning sensation that disappears with the increase in the anesthetic effect (within 1 minute), hyperemia. With spinal or epidural anesthesia, pain in the back, legs, partial/complete spinal blockade may be observed, accompanied by a decrease in blood pressure, impaired defecation, involuntary urination, impotence, loss of sensitivity in the perineum (the likelihood of these effects increases when using higher doses or in the case of accidental injection of lidocaine into the spinal space, when the dose intended for injection into the epidural space enters the spinal space); in some cases, after such an intervention, the restoration of motor, sensory and/or autonomic function occurs slowly (after several months) or incompletely.
Expiration date
3 years.
Storage conditions
Store in the original packaging at a temperature not exceeding 25°C.
Keep out of reach of children.
Incompatibility
The drug should not be mixed with other drugs in the same syringe. Lidocaine precipitates when mixed with amphotericin, methohexitone or sulfadiazine. Depending on the pH of the solution, lidocaine may be incompatible with ampicillin.
Packaging
2 ml in an ampoule; 5 ampoules in a film blister, 1 or 2 blisters in a pack.
2 ml in an ampoule; 10 ampoules in a cardboard pack with cardboard partitions.
Vacation category
According to the recipe.
Producer
JSC "Lubnypharm".
Location of the manufacturer and address of its place of business
Ukraine, 37500, Poltava region, Lubny, Barvinkova st., 16.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.