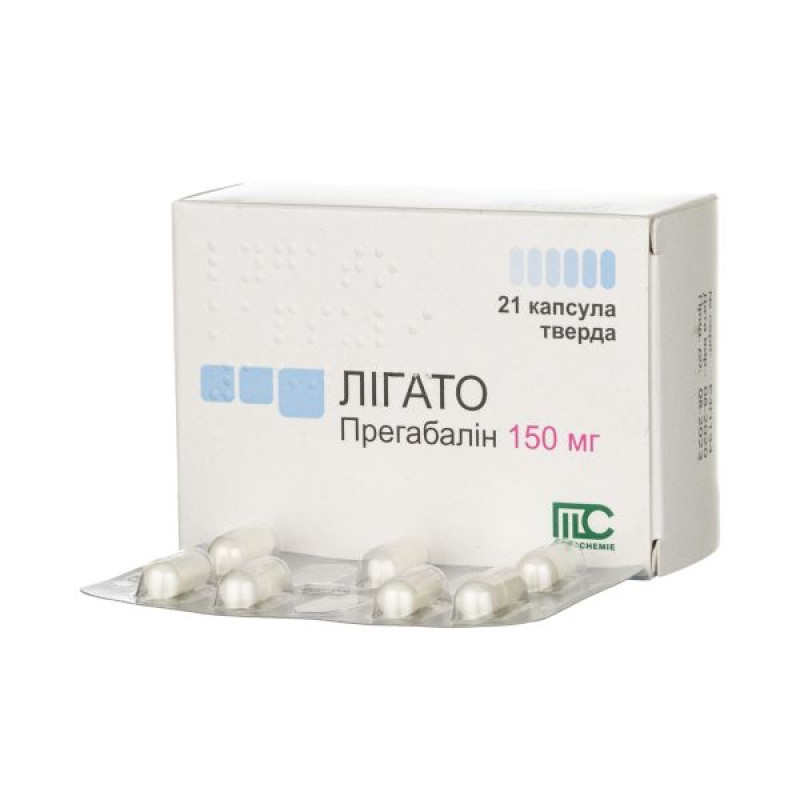
Ligato capsules 150mg No. 21

Ligato capsules are prescribed for the treatment of:
Neuropathic pain. The drug is indicated for the treatment of neuropathic pain of peripheral or central origin in adults. Epilepsy. The drug is indicated in adults as an adjunctive treatment for partial seizures with or without secondary generalization. Generalized anxiety disorder. The drug is indicated for the treatment of generalized anxiety disorder in adults.Composition
Active ingredient: pregabalin.
1 capsule contains 150 mg of pregabalin.
Excipients: lactose monohydrate; corn starch; talc; capsule shell: gelatin, titanium dioxide (E 171), patent blue V (E 131) (for 75 mg capsules); gelatin, titanium dioxide (E 171) (for 150 mg capsules); titanium dioxide (E 171), erythrosine (E 127), carmoisine (E 122), diamond blue FCF (E 133), gelatin (for 300 mg capsules).
Contraindication
Hypersensitivity to the active substance or to any of the excipients.
Method of application
The drug should be taken regardless of meals.
The dose range of the drug can vary within 150–600 mg per day. The daily dose should be divided into 2 or 3 doses.
Features
Pregnant women
There are no adequate data from the use of pregabalin in pregnant women.
Children
The safety and effectiveness of Ligato in children under 18 years of age have not been established.
Drivers
Pregabalin may have minor or moderate influence on the ability to drive and use machines.
Overdose
Post-marketing reports of overdose with pregabalin have included somnolence, confusion, agitation, and restlessness. Convulsions have also been reported. Coma has been reported rarely.
Treatment of pregabalin overdose consists of general supportive measures and may include hemodialysis if necessary.
Side effects
Infections and invasions.
Common: nasopharyngitis.
From the blood and lymphatic system.
Uncommon: neutropenia.
From the immune system.
Uncommon: hypersensitivity. Rare: angioedema, allergic reactions, anaphylactoid reactions.
Metabolic disorders, metabolism.
Common: increased appetite. Uncommon: loss of appetite, hypoglycaemia.
From the psychological side.
Common: euphoric mood, confusion, irritability, disorientation, insomnia, decreased libido. Uncommon: hallucinations, panic attacks, restlessness, agitation, depression, depressed mood, elevated mood, aggression, mood swings, depersonalization, difficulty in finding words, abnormal dreams, increased libido, anorgasmia, apathy. Rare: disinhibition.
From the nervous system.
Very common: dizziness, drowsiness, headache. Common: ataxia, coordination abnormal, tremor, dysarthria, amnesia, memory impairment, disturbance in attention, paraesthesia, hypoaesthesia, sedation, balance disorder, lethargy.
Uncommon: syncope, stupor, myoclonus, loss of consciousness, psychomotor hyperactivity, dyskinesia, dizziness postural, intention tremor, nystagmus, cognitive impairment, mental disorder, speech disorder, hyporeflexia, hyperaesthesia, burning sensation, ageusia, malaise, apathy, paraoral paraesthesia, myoclonus. Rare: convulsions, parosmia, hypokinesia, dysphagia, hypalgesia, dependence, cerebellar syndrome, cogwheel syndrome, coma, delirium, encephalopathy, extrapyramidal syndrome, Guillain-Barré syndrome, intracranial hypertension, manic reactions, paranoid reactions, sleep disorders.
From the organs of vision.
Common: blurred vision, diplopia, conjunctivitis. Uncommon: peripheral vision loss, visual impairment, eye swelling, visual field defects, visual acuity reduced, eye pain, asthenopia, photopsia, dry eye, lacrimation increased, eye irritation, blepharitis, accommodation disorder, ocular haemorrhage, photophobia, retinal oedema. Rare: vision loss, keratitis, oscillopsia, altered visual depth perception, mydriasis, strabismus, visual brightness, anisocoria, corneal ulcer, exophthalmos, ocular muscle paralysis, iritis, keratoconjunctivitis, miosis, night blindness, ophthalmoplegia, optic atrophy, optic disc oedema, ptosis, uveitis.
Interaction
Since pregabalin is excreted primarily unchanged in the urine, undergoes minimal metabolism in humans (≤ 2% of the dose is excreted in the urine as metabolites), does not inhibit the metabolism of other drugs in vitro, and is not bound to plasma proteins, it is unlikely that pregabalin could cause or be the target of pharmacokinetic interactions.
Storage conditions
Store in original packaging out of the reach of children.
Shelf life: 3 years.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.