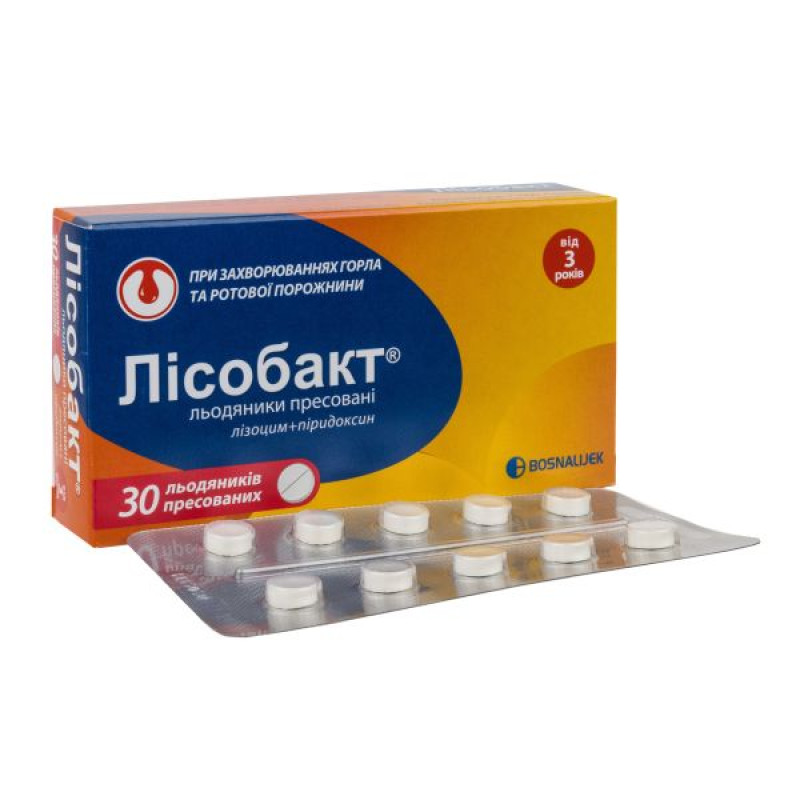
Lisobact pressed lollipops blister No. 30

Instructions Lisobact pressed lollipops blister No. 30
Composition
active ingredients: 1 pressed lollipop contains lysozyme hydrochloride 20 mg (not less than 720,000 OA FIP), pyridoxine hydrochloride 10 mg;
excipients: lactose monohydrate, tragacanth, magnesium stearate, sodium saccharin, vanillin.
Dosage form
The lollipops are pressed.
Main physicochemical properties: round lollipops with a diameter of 8 mm, white or almost white in color, with a smooth surface and a dividing line on one side.
Pharmacotherapeutic group
Drugs used for throat diseases. Antiseptics.
ATX code R02A A20.
Pharmacological properties
Pharmacodynamics.
Lysozyme is a mucopolysaccharide effective against gram-positive bacteria by converting insoluble cell wall polysaccharides into soluble mucopeptides. It is also effective against gram-negative bacteria, viruses and fungi. Lysozyme exhibits local anti-inflammatory activity and increases the body's nonspecific resistance. Pyridoxine has a protective effect on the oral mucosa and has an anti-aphthous effect.
Pharmacokinetics.
Data is missing.
Indication
Concomitant local treatment of diseases:
- oral mucosa, including aphthous stomatitis;
- throat: acute tonsillitis (angina), chronic tonsillitis, pharyngitis;
- in the postoperative period (after tonsillectomy, cryodestruction of the palatine tonsils).
Contraindication
Hypersensitivity to any component of the drug, including allergy to lysozyme or egg protein.
Interaction with other medicinal products and other types of interactions
LISOBACT should not be used simultaneously with other medicines containing antiseptics, as well as if other medicines containing pyridoxine hydrochloride are prescribed.
Lysozyme enhances the effectiveness of antibacterial drugs such as penicillin and chloramphenicol.
Isoniazid, penicillamine, pyrazinamide, hydralazine, immunosuppressants, estrogens (including oral contraceptives containing estrogens) may increase the need for pyridoxine because they act as pyridoxine antagonists or enhance the renal excretion of the latter.
Pyridoxine hydrochloride enhances decarboxylation and reduces the effectiveness of levodopa in the treatment of Parkinson's disease.
Application features
The medicine is not intended for long-term use.
In order to prevent disruption of the normal microflora of the oral cavity, the drug should not be used for more than 5 days.
This medicinal product contains lactose, therefore patients with rare hereditary forms of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicinal product. Patients who have been told by their doctor that they have an intolerance to some sugars should contact their doctor before taking this medicinal product.
If symptoms such as severe sore throat, headache, nausea, vomiting, high fever appear, or if the symptoms of the disease do not disappear within 5 days of treatment with the drug, you should consult a doctor.
In the case of a bacterial infection, it is necessary to consult a doctor regarding the appropriateness of using systemic antibiotic therapy.
The use of high doses of pyridoxine (more than 200 mg per day) for a long time (from several months to a year) may cause deterioration of the functional state of the liver and kidneys, sensory neuropathy, which usually disappears after discontinuation of treatment. Therefore, the use of the drug LISOBACT is not recommended for patients with impaired liver and kidney function and who are prescribed other drugs containing pyridoxine hydrochloride.
Since an increase in gastric acidity is possible when using pyridoxine in doses exceeding 30 mg/day, the drug should be used with caution in patients with peptic ulcer of the stomach and duodenum.
Use during pregnancy or breastfeeding
Pyridoxine: Adequate and well-controlled studies in pregnant women indicate no teratogenic effects or effects on fetal development.
Lysozyme: Animal studies have shown no teratogenic effects of lysozyme. There is insufficient data on the effects of lysozyme on the fetus. Therefore, use of the drug during pregnancy is not recommended.
Due to insufficient data on the excretion of lysozyme in breast milk and the high content of pyridoxine, the use of the drug during breastfeeding is not recommended.
Ability to influence reaction speed when driving vehicles or other mechanisms
Does not affect.
Method of administration and doses
The drug is intended for use by adults and children over 3 years of age.
Adults and children over 12 years of age: 2 lozenges 3–4 times a day.
Children aged 3 to 7 years: 1 lollipop no more than 3 times a day.
Children from 7 to 12 years old: 1 lollipop no more than 4 times a day.
Slowly dissolve the lozenges, keeping the resulting solution in your mouth for some time. The interval between doses is at least 1 hour. Duration of treatment is 5 days.
The medicine should not be used in children under 3 years of age.
Overdose
When using the drug LISOBACT in doses significantly exceeding the recommended ones, no clinically significant effects are expected.
The drug contains pyridoxine, so long-term use of pyridoxine (over 6–12 months) in doses exceeding 200 mg may lead to peripheral sensory neuropathy, which usually disappears after discontinuation of treatment.
Adverse reactions
On the part of the immune system: anaphylactic shock, anaphylactic reactions, angioedema.
Skin: urticaria, rash, itching, photosensitivity.
On the part of the digestive tract: sometimes the acidity of gastric juice increases.
Medical professionals, patients, and pharmacists are asked to report any suspected adverse reactions or lack of therapeutic effect to the email address of the Bosnalejek d.d. representative office: office@bosnalijek.com.ua.
Expiration date
5 years.
Storage conditions
Store out of the reach of children at a temperature not exceeding 30 ºС.
Packaging
10 pressed lollipops in a blister, No. 10 (10×1) or No. 30 (10×3) in a cardboard box.
Vacation category
Without a prescription.
Manufacturer/Applicant
Bosnalek, PhD
Location of the manufacturer and address of its place of business.
71000, Sarajevo, Jukiceva, 53, Bosnia and Herzegovina.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.