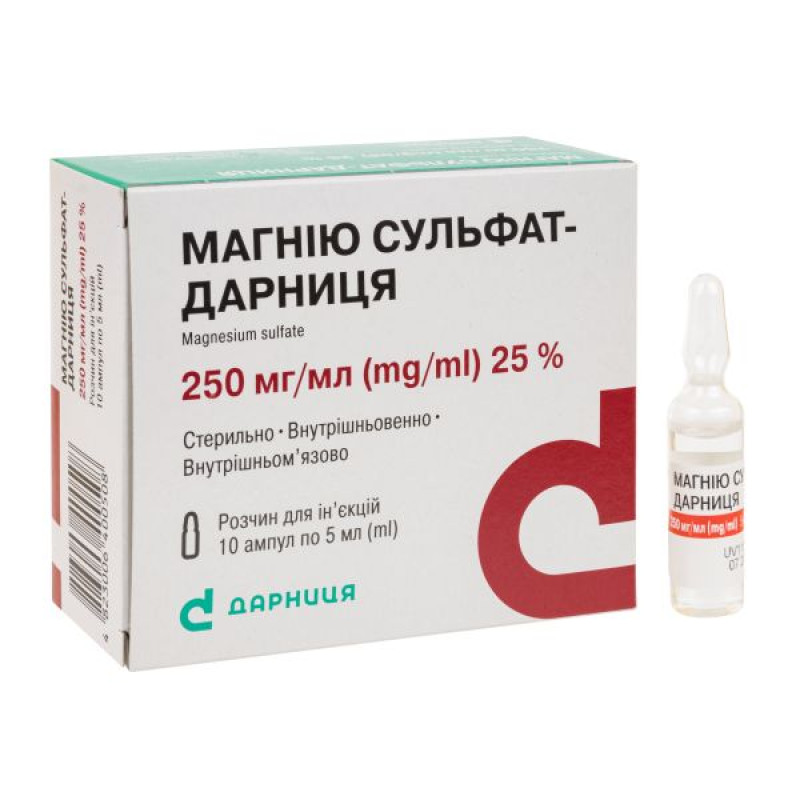
Magnesium sulfate-Darnitsa solution for injection 250 mg/ml ampoule 5 ml No. 10
Instructions for Magnesium sulfate-Darnitsa solution for injection 250 mg/ml ampoule 5 ml No. 10
Composition
active ingredient: magnesium sulfate heptahydrate;
1 ml of solution contains 250 mg of magnesium sulfate heptahydrate;
Excipients: water for injection, hydrochloric acid.
Dosage form
Solution for injection.
Main physicochemical properties: clear colorless liquid.
Pharmacotherapeutic group
Electrolyte solutions. ATX code B05X A05.
Pharmacological properties
Pharmacodynamics
When administered parenterally, it exhibits hypotensive, arteriolodilatory, antiarrhythmic, sedative, anticonvulsant, diuretic, antispasmodic, tocolytic effects. It replenishes magnesium deficiency in the body, is a physiological antagonist of calcium. It regulates metabolic processes, neurochemical transmission and muscle excitability, prevents the entry of calcium ions through the presynaptic membrane, reduces the amount of acetylcholine in the peripheral and central nervous system, exhibits sedative, hypnotic or narcotic effects depending on the dose, and has an antispasmodic effect. It reduces the excitability of the respiratory center; when administered in high doses, it can cause respiratory depression.
The hypotensive and antiarrhythmic effects of magnesium are due to a decrease in the excitability of cardiomyocytes, restoration of ionic balance, stabilization of cell membranes, disruption of sodium flow, slow-incoming calcium flow, and unilateral potassium flow, dilation of coronary arteries, reduction of total peripheral vascular resistance, platelet aggregation, as well as antispasmodic and sedative effects.
The sedative and anticonvulsant effects of magnesium are associated with a decrease in the release of acetylcholine from neuromuscular synapses, inhibition of neuromuscular transmission, and a direct inhibitory effect on the central nervous system.
Tocolytic action develops due to inhibition of the ability of the myometrium to contract (reduction of absorption, binding and distribution of calcium in smooth muscle cells), vasodilation and increased blood flow in the uterus. Magnesium exhibits antispasmodic action in urinary retention, is an antidote for poisoning with heavy metal salts.
Systemic effects develop almost instantly after intravenous and 1 hour after intramuscular administration, their duration is 30 minutes and 3-4 hours, respectively.
Pharmacokinetics
It passes through the blood-brain barrier and the placenta, is excreted in breast milk, the concentration in which is 2 times higher than the concentration in blood plasma. It is excreted by the kidneys, the rate of renal excretion is proportional to the concentration in blood plasma and the level of glomerular filtration. The concentration in blood plasma at which the anticonvulsant effect develops is 2-3.5 mmol/l.
Indication
Hypertensive crisis; ventricular arrhythmias (pirouette tachycardia); convulsive syndrome; eclampsia; hypomagnesemia; increased need for magnesium;
in the complex therapy of premature birth; angina pectoris; in poisoning with heavy metal salts; tetraethyl lead; soluble barium salts (antidote).
Contraindication
Increased individual sensitivity to the components of the drug; arterial hypotension, severe bradycardia (heart rate less than 55 beats/min), atrioventricular block, conditions caused by calcium deficiency and respiratory center depression, cachexia, renal dysfunction, severe hepatic or renal failure, myasthenia gravis, malignant neoplasms.
Interaction with other medicinal products and other types of interactions
Calcium ions have an antagonistic effect on magnesium ions, which leads to a decrease in the pharmacological effects of magnesium sulfate when used simultaneously. Enhances the effect of drugs that depress the central nervous system (narcotics, analgesics). With the simultaneous use of muscle relaxants and nifedipine, neuromuscular blockade is enhanced. Simultaneous use with calcium channel blockers, such as nifedipine, can lead to a violation of calcium balance and impaired muscle function.
Barbiturates, narcotic analgesics, and antihypertensives increase the likelihood of respiratory depression.
Cardiac glycosides increase the risk of developing conduction disturbances and atrioventricular block.
The effect of antithrombotic agents, vitamin K antagonists, isoniazid, and nonselective inhibitors of neuronal monoamine reuptake is reduced.
Mexiletine elimination may be delayed. Dose adjustment may be necessary.
Propafenone – enhances the effect of both drugs and increases the risk of toxic effects.
Disrupts the absorption of tetracycline antibiotics, intestinal obstruction is possible, and weakens the effect of streptomycin and tobramycin.
Application features
When using the drug, it should be taken into account that increased urinary magnesium excretion occurs with an increase in extracellular fluid, dilation of renal vessels, hypercalcemia, increased urinary sodium excretion, when prescribing osmotic diuretics (urea, mannitol, glucose), "loop" diuretics (furosemide, ethacrynic acid, thiazides), when taking cardiac glycosides, calcitonin, thyroidin, with prolonged administration of deoxycorticosterone acetate (more than 3-4 days). Slowing down the excretion of magnesium is observed with the administration of parathyroid hormone. In renal failure, magnesium excretion slows down, and with repeated administrations, its cumulation may occur. Therefore, in elderly patients and patients with severe renal impairment, the dose of the drug should not exceed 20 g of magnesium sulfate (81 mmol Mg2+) within 48 hours, patients with oliguria or severe renal impairment should not be given magnesium sulfate intravenously rapidly. Urinary tract infections accelerate the precipitation of ammonium-magnesium phosphates, and magnesium therapy is temporarily not recommended. In case of impaired magnesium excretion after parenteral administration of magnesium sulfate, hypermagnesemia is possible.
Use with caution in myasthenia gravis and respiratory diseases. With prolonged use of the drug, monitoring of the cardiovascular system, tendon reflexes, renal function and respiratory rate is recommended.
Intravenous administration of magnesium sulfate should be carried out slowly: if the rate of administration is too high, hypermagnesemia is possible (symptoms include nausea, paresthesias, sedation, hypoventilation up to apnea, and decreased deep tendon reflexes). Simultaneous parenteral administration of vitamin B6 and insulin increases the effectiveness of magnesium therapy.
If simultaneous intravenous administration of magnesium sulfate and calcium preparations is necessary, they should be administered into different veins, taking into account that the level of magnesium depends on the level of calcium in the body.
Use during pregnancy or breastfeeding
During pregnancy, magnesium sulfate should be used with extreme caution, taking into account the concentration of magnesium in the blood, in cases where the expected therapeutic effect outweighs the potential risk to the fetus. When providing analgesia during labor, the possibility of inhibiting the contractile ability of the uterine muscles should be taken into account, which requires the use of agents that stimulate labor.
If necessary, use of the drug should stop breastfeeding.
Ability to influence reaction speed when driving vehicles or other mechanisms
Patients should be warned about the need to be careful when working with potentially dangerous mechanisms or when driving vehicles, since the drug has a sedative effect.
Method of administration and doses
Administer intramuscularly, intravenously slowly or as an intravenous infusion. The frequency of administration and doses are individual depending on the indication and therapeutic effect. When administered by infusion, the drug is diluted with 0.9% sodium chloride solution or 5% glucose. When administered intravenously, the rate of administration should usually not exceed 150 mg/min (0.6 ml/min), except for the treatment of arrhythmias and eclampsia in pregnant women.
Hypomagnesemia. For moderately severe hypomagnesemia (0.5-0.7 mmol/L), administer 4 ml (1 g of magnesium sulfate) intramuscularly to adults every 6 hours.
In severe hypomagnesemia (< 0.5 mmol/l) with intramuscular administration, the total dose should be increased to 1 ml/kg (250 mg/kg) and administered in parts over 4 hours. In the form of intravenous infusion in severe hypomagnesemia, 20 ml of the drug (5 g of magnesium sulfate) should be added to 1 l of 0.9% sodium chloride solution or 5% glucose and administered over at least 3 hours.
The maximum daily dose for intravenous administration is 72 ml (18 g). If necessary, repeat the infusion over several days.
Arterial hypertension. In case of arterial hypertension of stage I-II, administer 5-10-20 ml intramuscularly daily. The course of treatment is 15-20 injections, while, along with a decrease in blood pressure, a decrease in the severity of angina may be observed.
Hypertensive crisis. Administer 10-20 ml intramuscularly or intravenously slowly.
Cardiac arrhythmias. To stop arrhythmias, administer 4-8 ml (1-2 g of magnesium sulfate) intravenously over 5-10 minutes, if necessary, repeat the injection (total administration of up to 4 g of magnesium sulfate).
It is possible to administer initially a loading dose of 8 ml over at least 5 minutes followed by infusion of 20 ml of the drug diluted in 0.9% sodium chloride solution or 5% glucose over at least 6 hours, or initially 8 ml over at least 30 minutes followed by infusion over at least 12 hours.
Ischemic stroke. 10-20 ml intravenously for 5-7 days.
Convulsive syndrome. Adults should be given 5-10-20 ml intramuscularly. Children should be given intramuscularly at the rate of 0.08-0.16 ml/kg (20-40 mg/kg).
In case of preeclampsia or eclampsia, administer intramuscularly or intravenously. First, inject 10 ml intramuscularly into each buttock once, or 16 ml (4 g of magnesium sulfate) intravenously over 3-4 minutes. Then continue to administer 16-20 ml (4-5 g) intramuscularly every 4 hours or intravenously drip 4-8 ml/hour (1-2 g/hour) with constant monitoring of tendon reflexes and respiratory function. Continue therapy until the attack stops. The maximum daily dose is 40 g of magnesium sulfate, in case of impaired renal function - 20 g/48 hours.
Labor pain relief. 5-10-20 ml intramuscularly, if necessary, combine magnesium sulfate with analgesics.
Urinary retention. For urinary retention and lead colic, administer 5-10 ml of the drug intramuscularly or 5-10 ml of a 25% solution of magnesium sulfate diluted 5 times intravenously (also administer as an enema).
As an antidote. In case of intoxication with mercury, arsenic, tetraethyl lead, intravenously administer 5-10 ml of a 25% solution of magnesium sulfate diluted 2.5-5 times. In case of poisoning with soluble barium salts, administer 4-8 ml intravenously or wash the stomach with a 1% solution of magnesium sulfate.
Newborns. For intracranial hypertension and severe asphyxia in newborns, administer intramuscularly, starting with a dose of 0.2 ml/kg per day, increasing the dose on the 3rd-4th day to 0.8 ml/kg per day, for 3-8 days in complex therapy. To eliminate magnesium deficiency in newborns, prescribe 0.5-0.8 ml/kg once a day for 5-8 days.
Children
The drug can be used in pediatric practice.
Overdose
Symptoms: signs of hypermagnesemia in order of increasing serum magnesium concentration:
decreased deep tendon reflexes (2-3.5 mmol/l);
prolongation of the PQ interval and expansion of the QRS complex on the ECG (2.5-5 mmol/l);
loss of deep tendon reflexes (4-5 mmol/l);
respiratory center depression (5-6.5 mmol/l);
cardiac conduction disorders (7.5 mmol/l);
cardiac arrest (12.5 mmol/l).
In addition, hyperhidrosis, anxiety, inhibition, polyuria, uterine atony.
Treatment: specific antidote is calcium preparations (calcium chloride or gluconate), which should be administered intravenously slowly. In case of moderate hypermagnesemia, furosemide may be prescribed. Respiratory depression should be eliminated by intravenous administration of 5-10 ml of 10% calcium chloride solution, oxygen inhalation, artificial ventilation of the lungs. In severe cases, peritoneal dialysis or hemodialysis is indicated. The appointment of symptomatic agents that correct the functions of the cardiovascular and central nervous systems.
Side effects
Respiratory, thoracic and mediastinal disorders: shortness of breath, respiratory depression.
Gastrointestinal: nausea, vomiting.
Renal and urinary disorders: polyuria.
From the side of metabolism: hypocalcemia, hypophosphatemia, hyperosmolar dehydration.
Nervous system: headache, dizziness, general weakness, drowsiness, confusion, loss of consciousness, decreased tendon reflexes, diplopia, speech disorders, tremor and numbness of the extremities.
From the psyche: depressed mood, anxiety.
From the cardiovascular system: arterial hypotension, bradycardia, palpitations, conduction disturbances, hot flashes, prolongation of the PQ interval and expansion of the QRS complex on the ECG, arrhythmia, coma, cardiac arrest.
Immune system disorders: hypersensitivity reactions, including anaphylactic shock, angioedema.
Skin and subcutaneous tissue disorders: hyperemia, itching, rash, urticaria.
Musculoskeletal and connective tissue disorders: muscle weakness.
From the reproductive system and mammary gland function: uterine atony.
General disorders and administration site reactions: hyperthermic syndrome, chills, increased sweating, hyperemia, edema, pain.
Expiration date
5 years.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 ° C. Do not freeze. Keep out of the reach of children.
Incompatibility
Pharmaceutically incompatible (precipitate is formed) with calcium preparations, ethanol (in high concentrations), carbonates, bicarbonates and phosphates of alkali metals, salts of arsenic acid, barium, strontium, clindamycin phosphate, hydrocortisone sodium succinate, polymyxin B sulfate, procaine hydrochloride, salicylates and tartrates. At Mg2+ concentrations above 10 mmol/ml in mixtures for total parenteral nutrition, the distribution of fat emulsions is possible.
Packaging
5 ml in an ampoule; 5 ampoules in a contour blister pack; 2 contour blister packs in a pack. 10 ml in an ampoule; 5 ampoules in a contour blister pack; 2 contour blister packs in a pack.
Vacation category
According to the recipe.
Producer
PrJSC "Pharmaceutical Company "Darnitsa".
Address
Ukraine, 02093, Kyiv, Boryspilska St., 13.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.