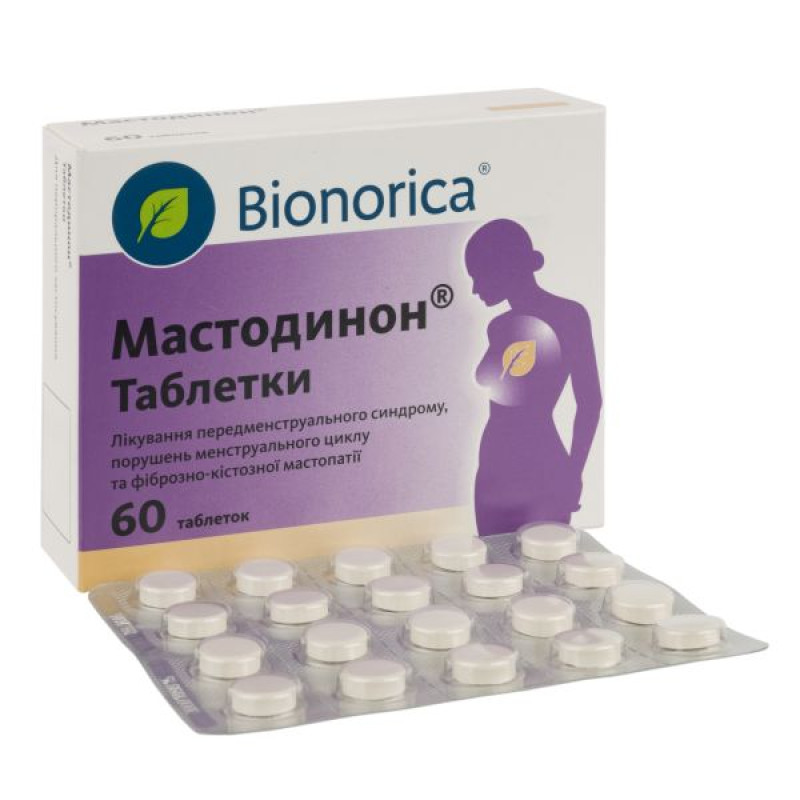
Mastodinon tablets blister pack No. 60
Instructions Mastodinon tablets blister No. 60
Composition
active ingredients: 1 tablet contains:
| Agnus castus Ø (castor tree) | 162 mg; |
| Saulorhullum thalictroides D4 (stem leaf basilicum-like) | 81 mg; |
| Cyclamen purpurascens D4 (purple violet) | 81 mg; |
| Strychnos ignatia D6 (Bitter Strychnos) | 81 mg; |
| Iris versicolor D2 (variegated iris) | 162 mg; |
| Lilium tigrinum D3 (tiger lily) | 81 mg; |
excipients: potato starch, lactose monohydrate, magnesium stearate.
Dosage form
Pills.
Main physicochemical properties: round, flat, beige tablets, inclusions are allowed.
Pharmacotherapeutic group
A complex homeopathic preparation.
Pharmacological properties
Pharmacodynamics
A drug for use in gynecological practice. Under the influence of the components of the drug, the concentration of prolactin in the blood decreases and a normalizing effect on the balance of sex hormones is manifested.
Pharmacokinetics
Data is missing.
Indication
In the complex treatment of premenstrual syndrome: mental lability, headache or migraine, edema, constipation, mastodynia (tenderness and tenderness of the mammary glands) before menstruation; menstrual cycle disorders and fibrocystic mastopathy.
Contraindication
Hypersensitivity to the components of the drug, including excipients. The drug should not be taken by patients with hereditary galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption.
Interaction with other medicinal products and other types of interactions
Interactions with dopamine agonists, dopamine antagonists, estrogens, and antiestrogens cannot be ruled out due to the possible dopaminergic and estrogenic effects of common primrose.
General remarks.
The effectiveness of homeopathic medicines can be negatively affected by an unhealthy lifestyle, consumption of stimulants and arousal agents (e.g., coffee).
If you are taking other medications, you should consult a doctor.
Application features
Patients who have or have had estrogen-dependent tumors should consult a doctor before using the drug Mastodinon, tablets.
The use of MastodinonÒ tablets in patients taking dopamine agonists, dopamine antagonists, estrogens and antiestrogens is possible only after consulting a doctor (see the section “Interaction with other medicinal products and other types of interactions”).
If symptoms worsen during treatment, you should consult a doctor.
Patients with a history of pituitary disorders should consult a doctor before using products containing common yam.
In the presence of prolactin-secreting pituitary tumors, the use of common thorn fruit may mask the symptoms of the tumor.
The drug Mastodinon® tablets should not be used before the onset of regular menstruation during the period of sexual development.
Note for diabetics. 1 tablet of Mastodinon® contains an average of 0.020 bread units. The medicine does not contain gluten.
Use during pregnancy or breastfeeding
There are no therapeutic indications for use during pregnancy, therefore the drug should not be used during pregnancy.
Reproductive studies suggest that Agnus castus may affect lactation. Use during lactation is not recommended.
Castor oil (Agnus castus) may affect a woman's ability to reproduce by regulating the menstrual cycle.
Ability to influence reaction speed when driving vehicles or other mechanisms
There is no information on the direct effect of the drug on driving or other mechanisms. In case of dizziness, you should refrain from driving or other mechanisms.
Method of administration and doses
Unless otherwise prescribed, take 1 tablet twice a day (morning and evening) with a small amount of water (e.g. a glass of water).
If symptoms persist or worsen while using the medicine, you should consult a doctor.
Children
There are no data on the use of this drug in children. Children and adolescents under 18 years of age should not use Mastodinon® tablets.
Overdose
When using large doses of the drug, which significantly exceed the recommended ones, gastrointestinal disorders or a laxative effect are possible in patients with lactose intolerance.
Treatment: symptomatic therapy.
Adverse reactions
The use of homeopathic medicines may lead to a temporary worsening of existing symptoms (initial exacerbation).
At the first signs of any adverse reactions, you should stop using the drug and consult a doctor.
Expiration date
4 years.
Do not use after the expiry date stated on the packaging.
The expiration date determines the use of the drug until the last day of the month.
Storage conditions
Store in original packaging out of the reach of children.
This medicinal product does not require any special temperature storage conditions.
Packaging
20 tablets in a blister, 3 (No. 60) or 6 (No. 120) blisters in a cardboard box.
Vacation category
Without a prescription.
Manufacturer/Applicant
Bionorica SE.
Location of the manufacturer and its business address/location of the applicant and/or the applicant's representative
Kerchensteinerstrasse, 11-15, 92318 Neumarkt, Germany.
Contact details of the manufacturer's representative in Ukraine, LLC "Bionorika":
phone: 044 521 86 00; fax: 044 521 86 01, info@bionorica.ua
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.