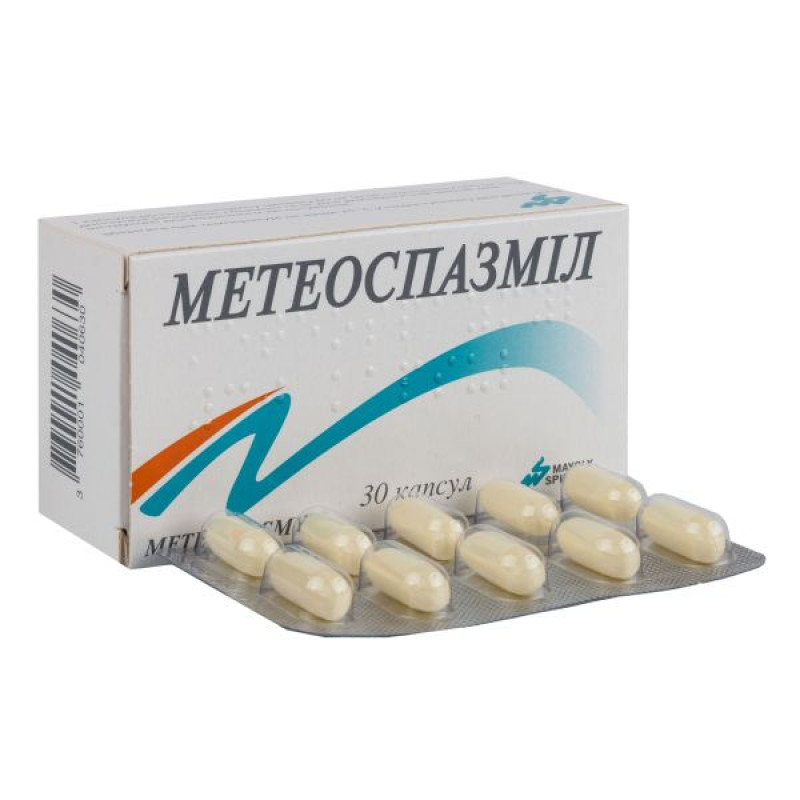
Meteospasmil capsules blister No. 30

Instructions for Meteospasmil capsules blister No. 30
Composition
active ingredients:
1 capsule contains alverine citrate 60 mg; simethicone 300 mg;
excipients: gelatin, glycerin, titanium dioxide (E 171).
Dosage form
Capsules.
Main physicochemical properties: opaque soft oblong capsules of pale yellow color, size No. 6.
Capsule contents: thick whitish suspension.
Pharmacotherapeutic group
Drugs used for functional disorders of the gastrointestinal tract. Alverin, combinations.
ATX code A03A X58.
Pharmacological properties
Pharmacodynamics
Alverine is a myotropic antispasmodic.
Simethicone is a physiologically inert substance with no pharmacological activity. It disrupts the surface tension of air bubbles, which promotes their coalescence.
Pharmacokinetics
After oral administration, simethicone is not adsorbed and is excreted unchanged after passing through the digestive tract.
Alverine is absorbed in the digestive tract and is rapidly converted into a pharmacologically active metabolite and inactive metabolites. After oral administration, peak plasma concentrations are reached in 1–1.5 hours. The main route of excretion of alverine metabolites is the kidneys.
Indication
Symptomatic treatment of functional bowel disorders, especially those manifested by flatulence.
Contraindication
Hypersensitivity to alverine or any other component of the drug.
Intestinal obstruction, including paralytic; obstructive diseases of the gastrointestinal tract.
Interaction with other medicinal products and other types of interactions
Not detected.
Application features
Liver function
Elevations of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels greater than 2 times the upper limit of normal (ULN) have been reported in patients treated with alverine/simethicone. This elevation may be associated with a concomitant increase in total serum bilirubin (see section 4.8). In the event of an increase in liver aminotransferases greater than 3 times the ULN or in the event of jaundice, Meteospasmil treatment should be discontinued.
Ability to influence reaction speed when driving vehicles or other mechanisms
Meteospasmil has a minor influence on the ability to drive or use machines. Adverse reactions such as dizziness have been observed in some patients (see sections "Adverse reactions" and "Overdose"). These types of disorders may affect the ability to drive or use machines.
Use during pregnancy or breastfeeding
Pregnancy
Simethicone: Due to the negligible systemic exposure to simethicone, no adverse effects are expected when used during pregnancy.
Alverine: There are no conclusive data on teratogenicity in animals. To date, no clinical effects such as malformations or fetotoxic effects have been reported. However, the observations on exposure to alverine during pregnancy are insufficient to exclude any risk.
Therefore, it is not recommended to use Meteospasmil during pregnancy.
Breast-feeding
Due to the low systemic exposure to simethicone, no negative effects are expected when used during breastfeeding.
There are no data on the excretion of alverine into human breast milk.
Therefore, it is not recommended to use Meteospasmil during breastfeeding.
Method of administration and doses
Use internally for adults, 1 capsule 2-3 times a day before meals or for pain.
The course of treatment is determined by the doctor individually.
Children
The drug is not used in pediatric practice.
Overdose
Dizziness has been reported when doses higher than recommended were used.
Adverse reactions
The following adverse reactions have been reported with the following frequencies: very common (≥ 1/10), common (≥ 1/100 and < 1/10), uncommon (≥ 1/1,000 and < 1/100), rare (≥ 1/10,000 and < 1/1,000), very rare (< 1/10,000) and not known (frequency cannot be estimated from the available data).
Nervous system: frequency unknown - headache.
On the part of the immune system: very rarely - anaphylactoid reactions, anaphylactic shock.
From the side of the organs of hearing and labyrinth: frequency unknown - dizziness.
Hepatobiliary disorders: very rarely – cytolytic hepatitis (see section "Special warnings and precautions for use").
Gastrointestinal: frequency unknown – nausea.
Skin and subcutaneous tissue disorders: frequency unknown - angioedema, skin rash, urticaria and itching.
Research results: frequency unknown - increased levels of transaminases, alkaline phosphatase and bilirubin.
Reporting suspected adverse reactions after a medicinal product has been authorised is important. This allows for continuous monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report suspected adverse reactions via the national reporting system.
Expiration date
3 years.
Storage conditions
Store at a temperature not exceeding 25 °C out of the reach of children.
Packaging
10 capsules in a blister, 3 blisters in a cardboard box.
Vacation category
Without a prescription.
Producer
Maioli Spindler Laboratories.
Location of the manufacturer and its business address
6, Avenue de l'Europe 78400 Chateaux France.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.