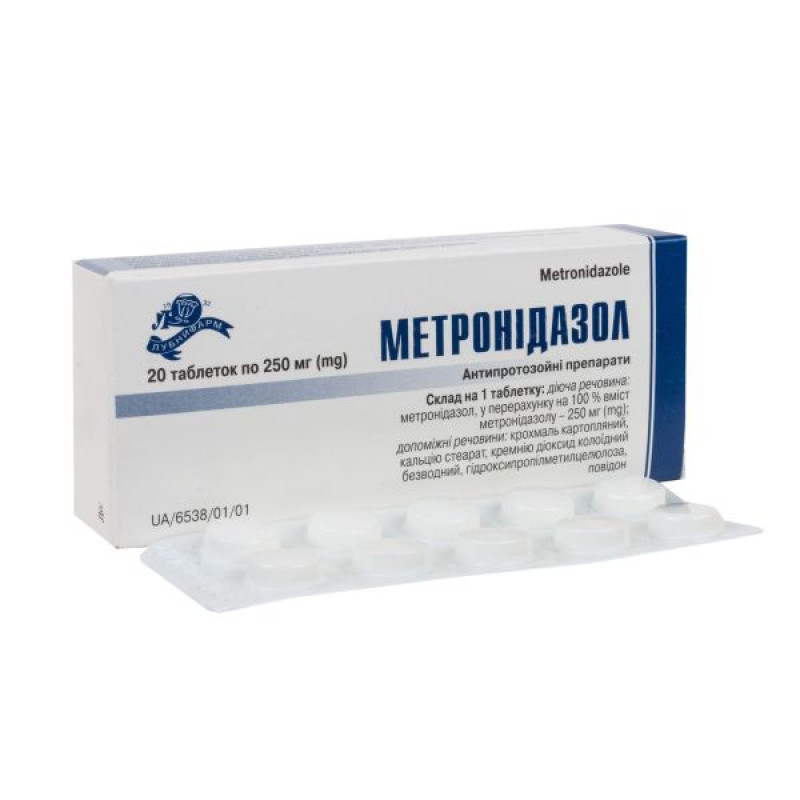
Metronidazole tablets 250 mg blister No. 20

Instructions Metronidazole tablets 250 mg blister No. 20
Composition
active ingredient: metronidazole;
1 tablet contains metronidazole, calculated as 100% metronidazole content 250 mg;
Excipients: potato starch, calcium stearate, colloidal anhydrous silicon dioxide, hydroxypropylmethylcellulose, povidone.
Dosage form
Pills.
Main physicochemical properties: solid, regular, round cylinders, the upper and lower surfaces of which are flat, the edges of the surfaces are beveled, with a dividing line, white or white with a yellowish or greenish tint.
Pharmacotherapeutic group
Antibacterials for systemic use. Antiprotozoal drugs. Imidazole derivatives. ATX code J01X D01.
Means for the treatment of amebiasis and other protozoal diseases. Antiprotozoal drugs. ATX code P01A B01.
Pharmacological properties
Pharmacodynamics
Metronidazole belongs to the nitro-5-imidazoles and has a broad spectrum of activity. The limit concentrations of the drug in the blood serum, which allow to differentiate sensitive strains (S) from strains with moderate sensitivity, and strains with moderate sensitivity from resistant strains (R), are as follows: S < 4 mg/l and R > 4 mg/l.
The prevalence of acquired resistance in certain species of microorganisms may vary with geographical location and time. Therefore, it is useful to have information on the local prevalence of resistance, especially when treating severe infections. These data are only a general guide to the likelihood of susceptibility of a particular bacterial strain to this antibiotic.
Sensitive to the drug: Peptostreptococcus spp., Clostridium spp., Bacteroides spp., Fusobacterium spp., Porphyromonas, Bilophila, Helicobacter pylori, Prevotella spp., Veilonella. Metronidazole inhibits the development of protozoa - Trichomonas vaginalis, Giardia intestinalis (Lamblia intestinalis), Entamoeba histolytica. Unstable sensitive to the drug: Bifidobacterium spp., Eubacterium spp. Insensitive strains of microorganisms: Propionibacterium, Actinomyces, Mobiluncus.
Pharmacokinetics
Absorption: Metronidazole is rapidly and almost completely absorbed after oral administration (at least 80% per hour). The maximum serum concentration achieved after oral administration is similar to that achieved after intravenous administration of equivalent doses.
Oral bioavailability is 100% and is not significantly reduced by concomitant food intake.
Distribution: Approximately 1 hour after a single 500 mg dose, the mean peak plasma concentration is 10 μg/mL. After 3 hours, the mean plasma concentration is 13.5 μg/mL.
The half-life is 8-10 hours, binding to blood proteins is insignificant - no more than 20%. The apparent volume of distribution is high (approximately 40 liters, i.e. 0.65 liters/kg).
Distribution is rapid and extensive, with concentrations reaching levels close to those in plasma, lungs, kidneys, liver, skin, bile, cerebrospinal fluid, saliva, seminal fluid, and vaginal secretions.
Metronidazole crosses the placental barrier and is excreted in breast milk.
Biotransformation. Metronidazole is metabolized by oxidation in the liver. Two metabolites are formed:
the main alcohol metabolite, which provides approximately 30% of the antibacterial activity of metronidazole against anaerobic bacteria, has a half-life of approximately 11 hours;
an acidic metabolite, which is present in smaller amounts and provides approximately 5% of the antibacterial activity of metronidazole.
Excretion. Significant concentration in the liver and bile; low concentration in the colon; insignificant elimination with feces. Excretion of the drug is carried out by 35-65% by the kidneys (in the form of metronidazole and oxidized metabolites).
Indication
Infections caused by microorganisms sensitive to the drug: amebiasis; urogenital trichomoniasis; nonspecific vaginitis; giardiasis; surgical infections caused by anaerobic microorganisms sensitive to metronidazole. As a replacement for intravenous treatment of infections caused by anaerobic microorganisms sensitive to metronidazole.
Contraindication
Hypersensitivity to metronidazole or to drugs of the imidazole group, as well as to other components of the drug. Cockayne syndrome (see section "Adverse reactions"). Children's age up to 6 years (which is due to the dosage form) (see section "Special instructions for use").
Interaction with other medicinal products and other types of interactions
Antabuse reaction
There are many medications that trigger an Antabuse reaction to alcohol, and their simultaneous use with alcohol is not recommended.
Combinations that are not recommended.
Alcohol (as a beverage or as an excipient in a medication). Antabuse effect (hot flashes, erythema, vomiting, tachycardia). Alcoholic beverages and medications containing alcohol should be avoided.
Disulfiram: Risk of acute psychotic episodes or confusion, which are reversible upon discontinuation of the drug.
Busulfan: When using busulfan in high doses: doubling of busulfan concentrations in patients receiving metronidazole.
Enzyme-inducing anticonvulsants: Decreased plasma concentrations of metronidazole due to increased hepatic metabolism by the enzyme inducer. Clinical monitoring is indicated and metronidazole dosage adjustment may also be necessary during and after treatment with the inducer.
Rifampicin: Decreased plasma concentrations of metronidazole due to increased hepatic metabolism by rifampicin. Clinical monitoring is indicated and metronidazole dosage adjustment may be necessary during and after rifampicin treatment.
Lithium: Increased lithium blood levels, which may be toxic, with signs of lithium overdose. Lithium blood levels should be monitored closely and dose adjustments may be necessary.
Combinations whose use requires special attention.
Fluorouracil (and, by extrapolation, tegafur and capecitabine). Increased toxicity of fluorouracil due to decreased clearance.
Special problems regarding the Ministry of Emergency Situations (international normalized ratio).
There have been numerous reports of increased activity of oral anticoagulants in patients receiving antibacterial therapy. Risk factors include the severity of the infection or inflammation, the patient's age, and the general health of the patient. In these circumstances, it is difficult to determine to what extent the MHC imbalance is caused by the infection itself or by its treatment. However, some classes of antibiotics are more likely to be involved in this effect, especially fluoroquinolones, macrolides, cyclines, co-trimoxazole, and some cephalosporins.
Application features
Hypersensitivity/skin and appendage disorders. Allergic reactions, including anaphylactic shock, which may be life-threatening, may occur (see section 4.8). In such cases, metronidazole treatment should be discontinued and appropriate therapy should be initiated.
If at the beginning of treatment the patient develops generalized erythema and pustular rashes accompanied by fever, acute generalized exanthematous pustulosis should be suspected (see section "Adverse reactions"); in the event of such a reaction, treatment with the drug should be discontinued, and further use of metronidazole, both alone and in combination with other drugs, is contraindicated.
Nervous system disorders: If symptoms suggestive of encephalopathy or cerebellar syndrome appear, the patient's treatment should be reviewed immediately and metronidazole should be discontinued.
Cases of encephalopathy have been reported during post-marketing surveillance. In addition, cases of MRI changes associated with encephalopathy have been observed (see section 4.8). The most common sites of involvement are the cerebellum (particularly the dentate nucleus) and the corpus callosum. In most cases, encephalopathy and MRI changes resolved after discontinuation of treatment. Fatalities have been reported very rarely.
Patients should be monitored for possible signs of encephalopathy or for worsening symptoms in patients with central nervous system disorders.
In the event of aseptic meningitis developing during treatment with the drug, re-administration of metronidazole is not recommended, and in patients with a serious infectious disease, a benefit/risk assessment should be performed.
Peripheral nervous system disorders: Patients should be monitored for signs of peripheral neuropathy, especially during long-term treatment or in the presence of severe, chronic, or progressive peripheral neurological disorders.
Psychiatric disorders: After receiving the first dose of the drug, patients may experience psychotic reactions that threaten the safety of patients, especially if they have a history of psychiatric disorders. If this occurs, metronidazole should be discontinued, the doctor should be informed and appropriate therapeutic measures should be initiated immediately.
Hematological effects: In patients with a history of hematological disorders or who receive the drug in high doses and/or for a long period, regular blood tests, especially for leukocyte counts, are necessary.
In patients with leukopenia, the decision to continue treatment with the drug depends on the severity of the infection.
Cases of severe hepatotoxicity/acute hepatic failure, including fatal cases with a very rapid course after the start of treatment in patients with Cockayne syndrome, have been reported with the use of metronidazole-containing medicinal products for systemic use. Therefore, metronidazole should not be used in this category of patients unless the benefit is considered to outweigh the risk and only if there is no alternative treatment. Liver function tests should be checked immediately before starting therapy, during treatment and after its termination until liver function tests are within normal limits or until baseline values are reached. If liver function tests increase markedly during treatment, the drug should be discontinued. Patients with Cockayne syndrome should be advised to immediately report any symptoms of potential liver damage to their doctor and to discontinue metronidazole (see section "Adverse reactions").
Pediatric patients. Tablets are contraindicated in children under 6 years of age due to the risk of aspiration. Other metronidazole formulations are available for young children.
Interaction with other medicinal products. The simultaneous use of metronidazole and alcohol is not recommended (see section "Interaction with other medicinal products and other types of interactions").
The simultaneous use of metronidazole and busulfan is not recommended (see section “Interaction with other medicinal products and other types of interactions”).
The simultaneous use of metronidazole and disulfiram is not recommended (see section "Interaction with other medicinal products and other types of interactions").
Effect on laboratory test results: Metronidazole may immobilize treponema, thereby causing a false-positive Nelson test result.
Use during pregnancy or breastfeeding
Pregnancy. Animal studies have not shown a teratogenic effect. Since no teratogenic effect has been observed in animals, no malformations are expected in humans. According to the data, substances that cause malformations in humans have a teratogenic effect in animals in adequately conducted studies in two species. From a clinical point of view, there was no fetotoxic effect on pregnancy after the analysis.
However, further epidemiological studies are needed to confirm the absence of risk. Therefore, metronidazole should be prescribed during pregnancy only if clearly needed, when the benefits of using the drug outweigh the potential risks.
Breastfeeding. Metronidazole passes into breast milk. Metronidazole should not be used during breast-feeding.
Ability to influence reaction speed when driving vehicles or other mechanisms
Patients should be aware of the possible occurrence of confusion, dizziness, hallucinations, seizures, or visual disturbances while taking the drug and should refrain from driving vehicles and operating other mechanisms during treatment.
Method of administration and doses
For amebiasis, take Metronidazole continuously for 7 days. Adults: 1.5 g per day, i.e. 500 mg (2 tablets) 3 times a day.
Children over 6 years of age: 30-40 mg/kg of body weight per day in 3 divided doses.
In the event of a liver abscess due to amebiasis, drainage or aspiration of pus should be performed simultaneously with metronidazole therapy.
Giardiasis should be treated for 5 days. Adults should be prescribed 750 mg-1 g of Metronidazole per day. Children aged 6-10 years - 375 mg/day, 10-15 years - 500 mg per day. To achieve the prescribed dosage, use metronidazole in the appropriate dosage or other dosage forms.
For trichomoniasis in women (urethritis and vaginitis caused by Trichomonas), Metronidazole is prescribed for a course of treatment for 10 days, combining 250 mg (1 tablet) 2 times a day and 1 vaginal suppository (500 mg) per day. The sexual partner should be treated at the same time, regardless of the presence or absence of clinical signs of Trichomonas infection, even if the result of laboratory tests is negative.
For trichomoniasis in men (urethritis caused by Trichomonas), Metronidazole is prescribed for a course of treatment for 10 days: 250 mg (1 tablet) 2 times a day.
In exceptional cases, it may be necessary to increase the daily dose to
750 mg or 1 g.
For nonspecific vaginitis, prescribe 500 mg (2 tablets) of the drug 2 times a day for 7 days. The sexual partner should be treated at the same time.
For the treatment of anaerobic infections (first-line therapy or replacement therapy), adults should be prescribed 1-1.5 g (4-6 tablets) of Metronidazole per day, and children over 6 years of age should be prescribed 20-30 mg/kg of body weight per day in 2 divided doses.
Children
The drug in the form of 250 mg tablets can be used in children aged 6 years and older.
Overdose
Single doses of up to 12 g have been observed in suicide attempts and accidental overdose.
Symptoms included vomiting, ataxia, and mild disorientation.
Treatment: There is no specific antidote. In case of significant overdose, symptomatic therapy should be used.
Side effects
Adverse reactions reported with metronidazole
minor gastrointestinal disorders (epigastric pain, nausea, vomiting, diarrhea);
glossitis with dry mouth, stomatitis, taste disturbances, anorexia;
pancreatitis, which is reversible after discontinuation of the drug;
discoloration or change in appearance of the tongue (mycosis).
On the part of the skin and its appendages:
hot flashes, itching of the skin, skin rash, which in some cases is accompanied by an increase in body temperature;
urticaria, angioedema, anaphylactic shock (see section "Special warnings and precautions for use");
very rare cases of acute generalized exanthematous pustulosis (see section "Special warnings and precautions for use").
toxic epidermal necrolysis;
fixed toxicoderma;
Stevens-Johnson syndrome.
From the nervous system:
peripheral sensory neuropathy;
headache;
dizziness;
cramps;
encephalopathy and subacute cerebellar syndrome (ataxia, dysarthria, gait disturbance, nystagmus, tremor), which may be accompanied by MRI changes that usually disappear after discontinuation of treatment with the drug. Very rarely, fatal cases have been reported (see section "Special instructions for use");
aseptic meningitis (see section "Special instructions").
On the part of the organs of vision:
temporary visual impairments, such as diplopia, myopia, blurred vision, decreased visual acuity, changes in color perception;
neuropathy/optic neuritis.
From the psyche:
hallucinations;
psychotic reactions with paranoia and/or delirium, which in some cases may be accompanied by suicidal thoughts or suicide attempts (see section "Special warnings and precautions for use");
depressed mood.
From the blood system:
neutropenia, agranulocytosis, thrombocytopenia.
From the hepatobiliary system:
Increased liver enzymes (AST, ALT, alkaline phosphatase), very rare cases of acute cholestatic or mixed hepatitis and hepatocellular liver damage, sometimes with jaundice, have been reported. Isolated cases of hepatocellular failure, which may require liver transplantation, have been reported.
Cases of severe irreversible hepatotoxicity/acute hepatic failure, including fatal cases, have been reported with very rapid onset after initiation of systemic metronidazole in patients with Cockayne syndrome (see section 4.3).
On the part of the hearing organs:
hearing impairment and hearing loss (including sensorineural);
tingle.
Others:
reddish-brown color of urine, caused by water-soluble pigments formed during the metabolism of the drug.
Expiration date
3 years.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 °C.
Keep out of reach of children.
Packaging
10 tablets in blisters.
10 tablets in a blister; 2 or 5 blisters in a pack.
Vacation category
According to the recipe.
Producer
JSC "Lubnypharm".
Address
Ukraine, 37500, Poltava region, Lubny, Barvinkova st., 16.
There are no reviews for this product.
There are no reviews for this product, be the first to leave your review.
No questions about this product, be the first and ask your question.